In short, you must replace the electrolyte when its chemical integrity is compromised. This is not based on a fixed time schedule but is instead determined by signs of contamination or degradation, which directly threaten the accuracy and reproducibility of your experimental results.
The decision to replace an electrolyte is an act of quality control. It's less about following a rigid schedule and more about recognizing when the electrolyte itself has become an uncontrolled variable in your experiment.

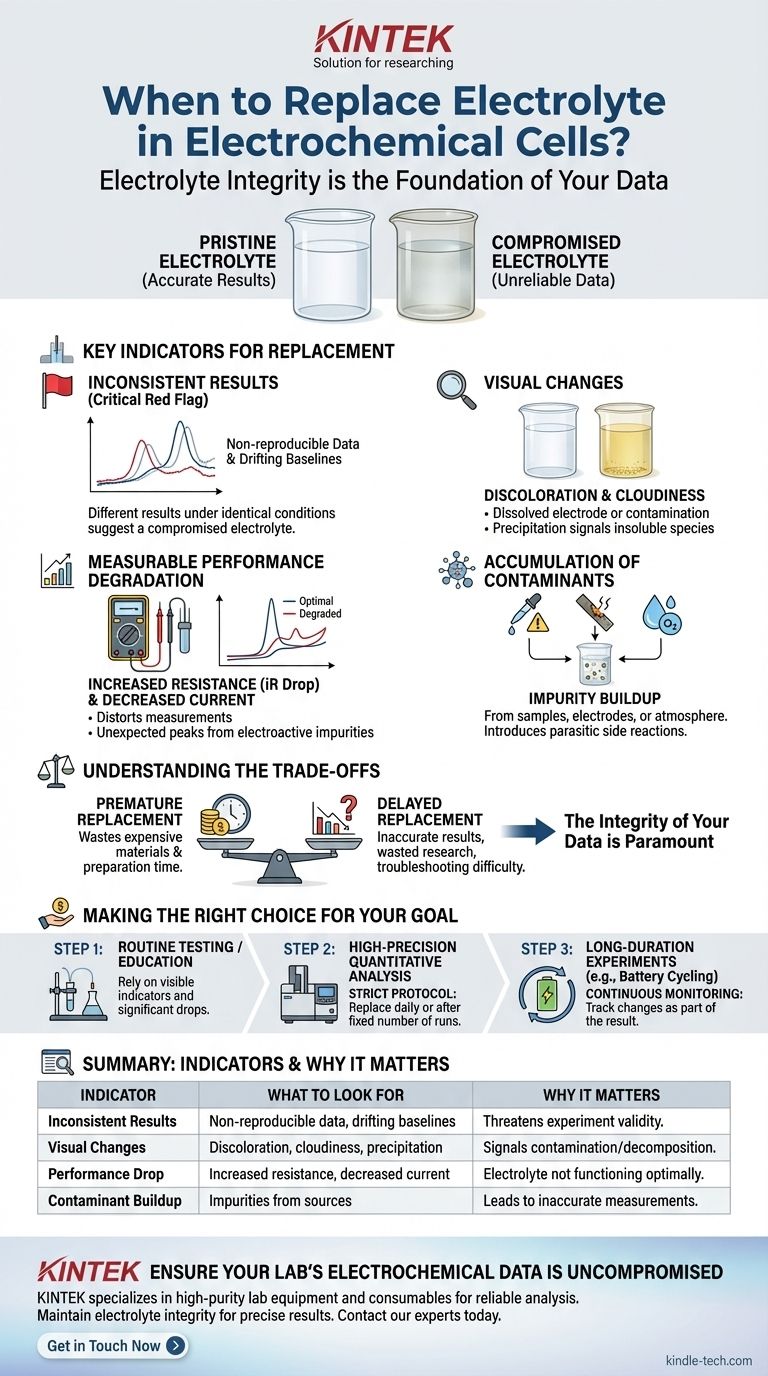
Why Electrolyte Integrity is the Foundation of Your Data
The electrolyte is not merely a passive medium; it is an active and critical component of any electrochemical cell. Its primary role is to transport ions between the electrodes, completing the electrical circuit.
Any change in its composition, purity, or concentration can fundamentally alter the behavior of your system. This directly impacts the validity of the data you collect.
The Key Indicators for Electrolyte Replacement
Degradation is not a single event but a gradual process. You must learn to recognize the signs that indicate the electrolyte is no longer suitable for use.
Inconsistent or Non-Reproducible Results
This is the most critical red flag. If you run the same experiment under identical conditions and get different results, a compromised electrolyte is a primary suspect. Look for drifting baselines or shifting peak potentials between runs.
Visual Changes in the Solution
Your eyes are a powerful first-line tool. A pristine electrolyte is typically clear and colorless.
Be alert for discoloration, which can indicate the dissolution of an electrode or reactions with contaminants. Also, watch for cloudiness or precipitation, which signals that insoluble species are forming.
Measurable Performance Degradation
Your electrochemical data will often reveal a failing electrolyte. A common sign is a noticeable increase in the cell's internal resistance (iR drop), which can distort measurements.
You might also observe a general decrease in current or the appearance of small, unexpected peaks in your voltammograms, which can be caused by electroactive impurities.
Accumulation of Contaminants
Every experiment introduces potential impurities into the electrolyte. These can come from the sample you are analyzing, slow dissolution of the electrodes, or even absorption of water and oxygen from the atmosphere.
Over time, these impurities build up and can introduce parasitic side reactions that interfere with the measurement you care about.
Understanding the Trade-offs
Deciding when to replace the electrolyte involves balancing cost and convenience against the risk of generating bad data.
The Cost of Premature Replacement
Electrolyte solutions, especially those using high-purity solvents and salts or specialized ionic liquids, can be expensive. Constantly replacing a solution that is still perfectly viable wastes both materials and the time required for preparation.
The High Risk of Delayed Replacement
The consequences of using a degraded electrolyte are far more severe. It leads to inaccurate results, wasted research time spent on invalid experiments, and immense difficulty in troubleshooting unexpected outcomes. The integrity of your data is paramount.
Making the Right Choice for Your Goal
Your replacement strategy should be adapted to the sensitivity of your work.
- If your primary focus is on routine testing or educational demonstrations: You can rely mainly on visible indicators like discoloration or significant performance drops before replacement.
- If your primary focus is on high-precision quantitative analysis: You must adopt a strict replacement protocol. Consider replacing the electrolyte daily or after a small, fixed number of experiments, even without visible signs of degradation.
- If your primary focus is on long-duration experiments (e.g., battery cycling): Monitor key performance metrics continuously. Replacement may not be an option, but tracking changes attributed to the electrolyte is part of the experimental result itself.
Proactively managing your electrolyte's health is the simplest way to ensure your electrochemical data is reliable and trustworthy.
Summary Table:
| Indicator | What to Look For | Why It Matters |
|---|---|---|
| Inconsistent Results | Non-reproducible data, drifting baselines | Indicates compromised chemical integrity, threatening experiment validity. |
| Visual Changes | Discoloration, cloudiness, precipitation | Signals contamination or decomposition of the electrolyte. |
| Performance Drop | Increased resistance, decreased current, unexpected peaks | Shows the electrolyte is no longer functioning optimally. |
| Contaminant Buildup | Impurities from samples, electrodes, or atmosphere | Leads to parasitic side reactions and inaccurate measurements. |
Ensure Your Lab's Electrochemical Data is Uncompromised
Don't let a degraded electrolyte undermine your research. KINTEK specializes in high-purity lab equipment and consumables essential for reliable electrochemical analysis. From solvents and salts to specialized cells, we provide the quality materials you need to maintain electrolyte integrity and achieve precise, reproducible results.
Contact our experts today to discuss your laboratory's specific needs and ensure the foundation of your data is solid.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments



















