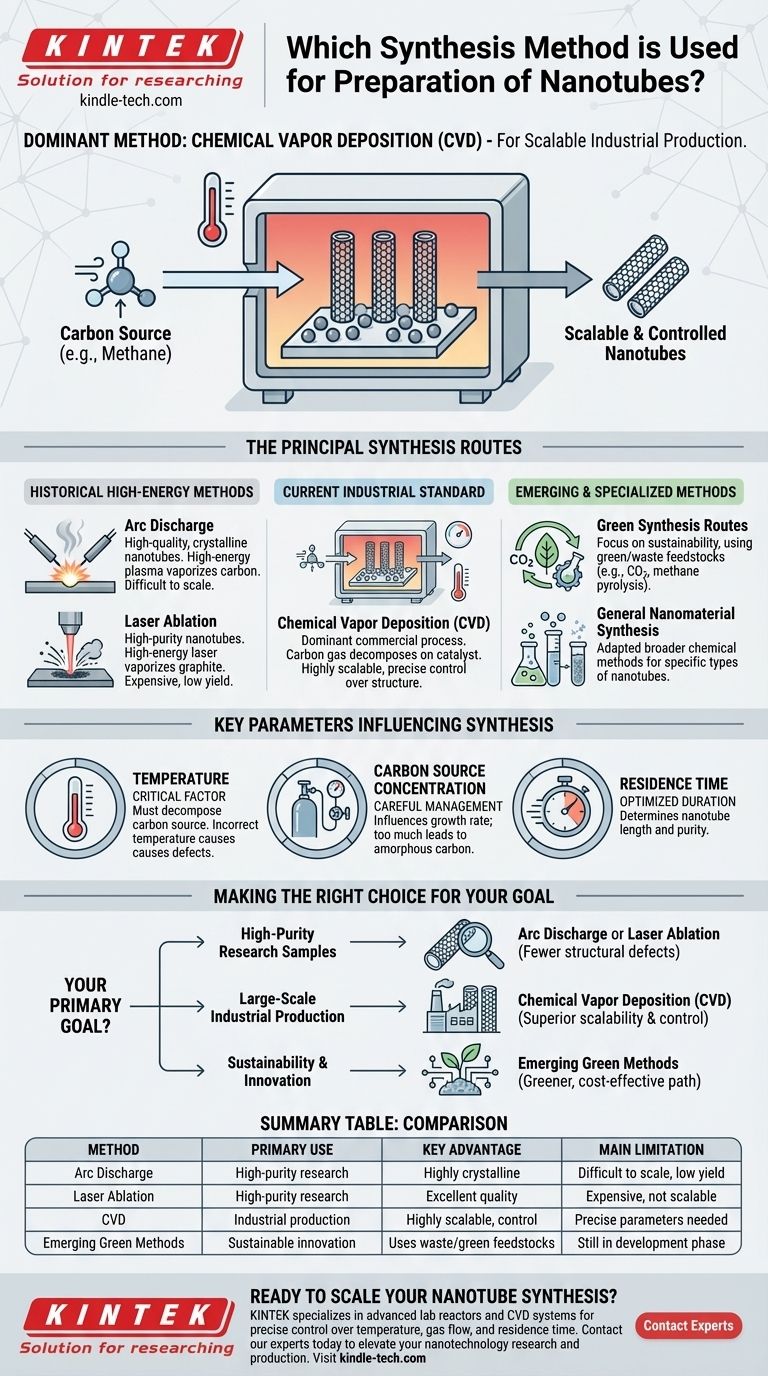
Several synthesis methods are used for preparing nanotubes, but the most dominant for commercial production is Chemical Vapor Deposition (CVD). While traditional methods like arc discharge and laser ablation were foundational, CVD offers the scalability and control required for industrial applications. Emerging techniques are also being developed with a focus on sustainability.
While early, high-energy methods can produce high-quality nanotubes, Chemical Vapor Deposition (CVD) has become the industry standard. This is due to its superior scalability and control over the final product's structure, which are critical for commercial viability.

The Principal Synthesis Routes for Nanotubes
Understanding the different methods for creating nanotubes requires looking at them in terms of their historical development and their specific applications. They generally fall into three categories: historical high-energy methods, the current industrial standard, and emerging sustainable approaches.
Arc Discharge
The arc discharge method was one of the first techniques used to produce carbon nanotubes. It involves creating a high-temperature plasma arc between two graphite electrodes.
This high-energy process vaporizes the carbon, which then condenses to form nanotubes. It is known for producing high-quality, highly crystalline nanotubes but is difficult to scale for mass production.
Laser Ablation
Similar to arc discharge, laser ablation uses a high-energy source—in this case, a laser—to vaporize a graphite target in a high-temperature furnace.
The resulting carbon vapor cools and condenses on a collector. This method also produces high-purity nanotubes but is expensive and has low yield, limiting its use primarily to research environments.
Chemical Vapor Deposition (CVD)
CVD is the dominant commercial process for nanotube synthesis today. This method involves introducing a carbon-containing gas (like methane or acetylene) into a high-temperature furnace with a catalyst.
The hydrocarbon decomposes at high temperatures, and the carbon atoms deposit onto the catalyst particles, growing into nanotubes. CVD is favored because it allows for greater control over the nanotube's length, diameter, and alignment, and it is far more scalable than older methods.
Emerging and Specialized Methods
As the field matures, new methods are being developed to address the cost and environmental impact of traditional synthesis.
Green Synthesis Routes
Emerging methods are exploring the use of green or waste feedstocks. This includes innovative approaches like using carbon dioxide captured by electrolysis in molten salts or using methane pyrolysis to produce both nanotubes and valuable hydrogen gas.
General Nanomaterial Synthesis
Broader chemical synthesis techniques, such as hydrothermal methods and sol-gel methods, are also used to prepare various types of nanomaterials. While more commonly associated with other nanostructures, these can be adapted for specific types of nanotubes, particularly non-carbon-based ones.
Key Parameters Influencing Synthesis
The success of any synthesis method, especially CVD, depends on precise control over several operating parameters. These variables directly influence the quality, yield, and type of nanotubes produced.
The Role of Temperature
Temperature is a critical factor. It must be high enough to decompose the carbon source and facilitate nanotube growth on the catalyst, but incorrect temperatures can lead to defects or unwanted carbon byproducts.
Carbon Source Concentration
The concentration of the carbon-containing gas must be carefully managed. Too little will result in a slow growth rate, while too much can deactivate the catalyst and lead to the formation of amorphous carbon instead of well-structured nanotubes.
Residence Time
Residence time refers to how long the carbon gas stays within the reaction zone. This parameter significantly influences the length and purity of the nanotubes and must be optimized for efficient production.
Making the Right Choice for Your Goal
Selecting a synthesis method is not about finding the "best" one in absolute terms, but the most appropriate one for a specific objective.
- If your primary focus is high-purity research samples: Arc discharge or laser ablation are often preferred for producing nanotubes with fewer structural defects.
- If your primary focus is large-scale industrial production: Chemical Vapor Deposition (CVD) is the undisputed standard due to its superior scalability, lower cost, and process control.
- If your primary focus is sustainability and innovation: Exploring emerging methods like methane pyrolysis offers a path toward greener and more cost-effective nanotechnology.
Ultimately, the optimal synthesis method is defined by the specific balance of quality, quantity, and cost required for your application.
Summary Table:
| Method | Primary Use | Key Advantage | Main Limitation |
|---|---|---|---|
| Arc Discharge | High-purity research | Produces highly crystalline nanotubes | Difficult to scale, low yield |
| Laser Ablation | High-purity research | Excellent nanotube quality | Expensive, not scalable |
| Chemical Vapor Deposition (CVD) | Industrial production | Highly scalable, excellent process control | Requires precise parameter optimization |
| Emerging Green Methods | Sustainable innovation | Uses waste/green feedstocks | Still in development phase |
Ready to scale your nanotube synthesis?
Whether you are optimizing a research process or scaling up for industrial production, the right equipment is critical. KINTEK specializes in advanced lab reactors and CVD systems designed for precise control over temperature, gas flow, and residence time—the key parameters for successful nanotube growth.
Our expertise in lab equipment and consumables can help you achieve higher yields and superior quality. Let's discuss your specific application and build a solution tailored to your goals.
Contact our experts today to elevate your nanotechnology research and production.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What is the vapor phase deposition technique? A Guide to PVD & CVD Thin-Film Coating Methods
- What are the steps of the CVD process? A Guide to Precision Thin Film Deposition
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings



















