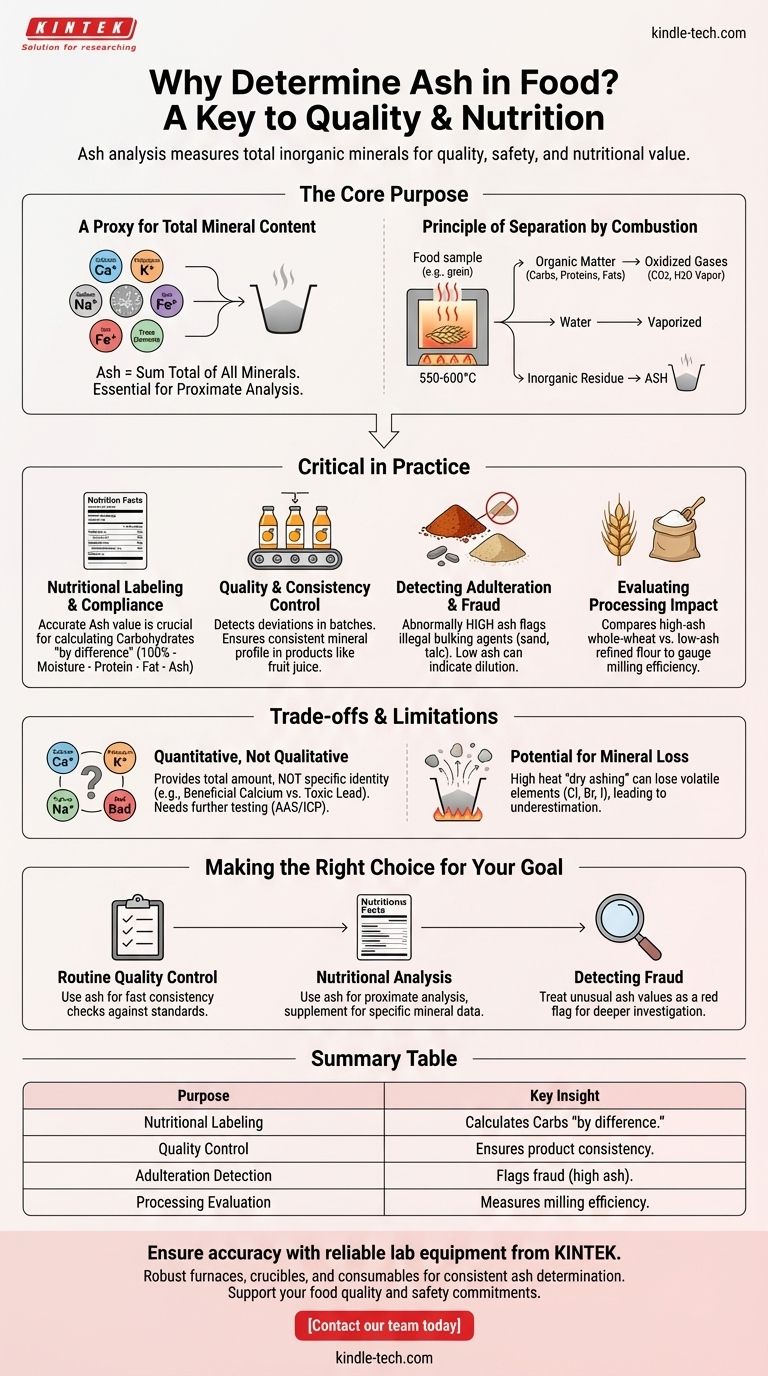
Determining the ash content of food is a foundational analytical procedure used to measure the total amount of inorganic minerals within a product. This single value serves as a critical indicator of nutritional content, product quality, and authenticity. The process involves incinerating a sample at high temperatures to burn off all organic matter—like carbohydrates, proteins, and fats—leaving behind only the non-combustible mineral residue, known as ash.
While ash analysis seems like a simple measure of what remains after burning, it is actually a powerful diagnostic tool. It provides a crucial, high-level view of a food's nutritional profile, processing integrity, and overall quality.
The Core Purpose of Ash Analysis
A Proxy for Total Mineral Content
The term "ash" is a direct reference to the inorganic residue left after complete combustion. This residue is composed of essential minerals required by the body, such as calcium, potassium, sodium, and iron, as well as trace elements.
Ash content gives you a single, quantitative value representing the sum total of all these minerals. It is a fundamental component of "proximate analysis," the standard suite of tests that breaks down a food into its major constituents (moisture, protein, fat, carbohydrates, and ash).
The Principle of Separation by Combustion
The analysis works on a simple principle: organic matter is combustible, while minerals are not.
By subjecting a food sample to very high temperatures (typically 550-600°C), all water is vaporized, and organic compounds are oxidized into gases like carbon dioxide and water vapor. What remains in the crucible is the inorganic, mineral fraction of the food.
Why This Measurement is Critical in Practice
Nutritional Labeling and Compliance
Ash measurement is a cornerstone of nutritional science. The nutrition facts panel on food packaging is derived from proximate analysis.
Since carbohydrates are difficult to measure directly, their value is often calculated "by difference." This is done by subtracting the measured percentages of moisture, protein, fat, and ash from 100%. An accurate ash value is therefore essential for an accurate carbohydrate declaration.
Quality and Consistency Control
For food manufacturers, ash content is a vital quality control specification. A fruit juice, for example, should have a consistent mineral profile from its source fruit.
If a batch of juice shows an ash content that deviates significantly from the established standard, it can indicate a problem with the raw ingredients, a dilution issue, or an error in the manufacturing process.
Detecting Adulteration and Fraud
Ash analysis is a classic method for detecting certain types of economic adulteration. For instance, ground spices might be illegally bulked up with sand, brick dust, or talc to increase weight and profit.
These inorganic adulterants are non-combustible and would cause an abnormally high ash value, immediately flagging the product as suspicious. Similarly, if a premium product like whole-wheat flour is diluted with low-ash refined flour, the resulting ash content will be lower than specified.
Evaluating Processing Impact
The mineral content of food is often concentrated in specific parts, such as the bran and germ of a grain. The process of milling wheat into refined white flour removes these components.
By comparing the ash content of whole-wheat flour (high ash) to refined flour (low ash), a miller can precisely gauge the efficiency and degree of the milling process.
Understanding the Trade-offs and Limitations
It's a Quantitative, Not Qualitative, Measure
The most significant limitation of ash analysis is that it provides a total quantity but gives no information about the identity of the specific minerals present.
A simple ash test cannot distinguish between beneficial calcium and toxic heavy metals like lead. It only tells you the total sum of all inorganic material. For specific mineral identification, more advanced methods like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma (ICP) are required.
Potential for Mineral Loss During Analysis
The standard "dry ashing" method involves very high heat, which can cause certain volatile minerals to be lost. Elements like chlorine, bromine, and iodine can vaporize and escape, leading to an underestimation of the true mineral content.
Alternative methods like "wet ashing" or "low-temperature ashing" can mitigate this, but the choice of method must be appropriate for the food matrix and the specific analytical goal.
Making the Right Choice for Your Goal
The value you derive from an ash measurement depends entirely on the question you are trying to answer.
- If your primary focus is routine quality control: Use ash content as a fast and reliable index to verify product consistency against a known standard.
- If your primary focus is nutritional analysis: Treat ash as a foundational part of proximate analysis, but supplement it with more specific techniques to quantify individual essential minerals and create a complete nutritional profile.
- If your primary focus is detecting fraud: View an unusually high or low ash value as a critical red flag that warrants a deeper investigation into potential adulteration or mislabeling.
Ultimately, understanding the context behind the ash value transforms it from a simple number into a powerful diagnostic tool for ensuring food quality and safety.

Summary Table:
| Purpose of Ash Analysis | Key Insight |
|---|---|
| Nutritional Labeling | Provides the mineral content value needed to accurately calculate carbohydrates "by difference." |
| Quality Control | Serves as a benchmark to ensure product consistency and detect deviations in manufacturing. |
| Adulteration Detection | Flags potential fraud (e.g., added sand or talc) through abnormally high ash values. |
| Processing Evaluation | Measures the efficiency of processes like milling by comparing ash levels in different flour types. |
Ensure the accuracy and integrity of your food analysis with reliable lab equipment from KINTEK.
Whether you are performing proximate analysis for nutritional labeling, maintaining strict quality control standards, or screening for adulterants, the precision of your ash determination is critical. KINTEK specializes in supplying robust and accurate laboratory furnaces, crucibles, and consumables designed for consistent, high-temperature ashing procedures.
Let our expertise in lab equipment support your commitment to food quality and safety. Contact our team today to find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the function of a high-precision high-temperature furnace for 18 Ni (300) steel? Ensure Optimal Microstructure
- What role does a muffle furnace play in ZnO powder synthesis? Master Two-Stage Heat Treatment Success
- What is the difference between a muffle furnace and an electric furnace? A Guide to Precision Heating
- What is the primary function of a high-temperature furnace in KIT-6 preparation? Unlock Mesoporous Silica Potential
- Why is a high-temperature muffle furnace essential for perovskite synthesis? Master Solid-State Reactions
- How hot can a muffle furnace get? Find the Right Temperature for Your Lab
- What is the inside material of the muffle furnace? Discover the Refractory Core for High-Temp Precision
- What role does a box resistance furnace play in the pretreatment of coal gangue? Enhance ZSM-5 Zeolite Synthesis Results



















