Introduction to PECVD and Plasma
Definition and Function of Plasma in PECVD
Plasma Enhanced Chemical Vapor Deposition (PECVD) harnesses the power of plasma to significantly enhance the deposition process. Plasma, a highly ionized gas, is typically generated using a radio frequency (RF) current or through high-energy electron-activated alternating current (AC) or direct current (DC) discharges between two parallel electrodes. This ionized state of gas is crucial as it provides the necessary energy to intensify the thermal movement of material molecules, leading to their ionization and the formation of a complex mixture comprising positive ions, electrons, and neutral particles.
In a PECVD setup, the plasma is not merely a byproduct but an active participant in the deposition process. Operating under vacuum conditions, often at pressures below 0.1 Torr, PECVD allows for the deposition of thin films at relatively low substrate temperatures, ranging from room temperature to 350°C. This is a significant advantage over conventional Chemical Vapor Deposition (CVD) methods, which often require higher temperatures to drive the necessary chemical reactions. By leveraging plasma, PECVD can achieve these reactions at lower temperatures, reducing thermal stress on the substrate and enhancing the bonding strength of the deposited films.
The primary role of plasma in PECVD is to promote and sustain chemical reactions. The electrons within the plasma, which can have energies ranging from 1 to 20 eV, create a highly reactive environment. These energetic electrons are capable of ionizing and dissociating most gas molecules, forming reactive species such as free radicals that can interact with the substrate surface. This interaction results in the modification and coating of the substrate surface, enhancing the overall deposition efficiency. Additionally, the high-energy ultraviolet (UV) photons generated within the plasma can further activate the substrate, creating more reactive sites and facilitating the deposition process.
This combination of low-temperature operation, efficient reaction promotion, and enhanced deposition rates makes PECVD a versatile and powerful technique for thin film deposition in various applications.
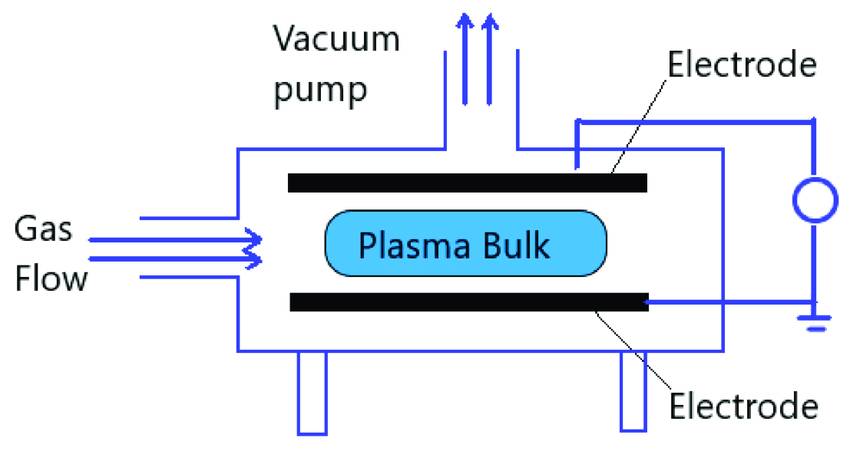
PECVD System Components
A PECVD system is a sophisticated apparatus designed to facilitate the deposition of thin films on substrates through a series of intricate processes. At its core, the system includes generators that employ graphite boats and high-frequency plasma exciters to create the necessary conditions for chemical reactions. The plasma generator is strategically positioned in the middle of the coated plate, where it operates under low pressure and elevated temperatures to initiate and sustain the reaction.
In typical semiconductor applications, the substrate is placed within a deposition chamber that houses two parallel electrodes: a ground electrode and an RF-energized electrode. This setup allows for precise control over the electrical discharge that ignites the plasma. Precursor gases, such as silane (SiH4) and ammonia (NH3), are often mixed with inert gases like argon (Ar) or nitrogen (N2) to fine-tune the process. These gases are introduced into the chamber via a showerhead fixture above the substrate, ensuring an even distribution that enhances the uniformity of the deposited film.
The plasma is ignited by an electric discharge between the electrodes, typically ranging from 100 to 300 eV. This discharge generates the thermal energy required to drive the chemical reactions that lead to the growth of the film. The precursor gas molecules, energized by collisions with high-energy electrons, propagate through the gas flow to the substrate. Once there, they react and are absorbed onto the substrate surface, forming the desired film. The chemical byproducts of these reactions are then efficiently removed from the chamber, completing the deposition process.
The PECVD equipment shares similarities with Physical Vapor Deposition (PVD) systems, including a chamber, vacuum pumps, and a gas distribution system. However, the configuration differences primarily lie in the power source, gas types and flow levels, pressure sensors, and the overall design of the parts racking. Hybrid systems, capable of performing both PVD and PECVD, offer the flexibility to leverage the strengths of both processes. While PVD is generally a line-of-sight process, PECVD produces coatings that tend to cover all surfaces within the chamber, necessitating different maintenance and utilization strategies based on the specific process requirements.
Role of Graphite Boats in PECVD
Electrical and Thermal Conductivity of Graphite
Graphite boats play a pivotal role in Plasma Enhanced Chemical Vapor Deposition (PECVD) processes, primarily due to their exceptional electrical and thermal conductivity. These properties enable graphite boats to efficiently manage the complex interactions within the PECVD system, ensuring the precise deposition of coatings.
When an alternating current (AC) voltage is applied, graphite boats create distinct positive and negative poles. This polarity differentiation is crucial as it initiates a phenomenon known as glow discharge. During glow discharge, the electrical energy is converted into kinetic energy, which accelerates the movement of gas molecules. This increased kinetic activity leads to the ionization of silane (SiH4) and ammonia (NH3) gases, breaking them down into their constituent elements—silicon (Si) and nitrogen (N) ions.
The thermal conductivity of graphite boats is equally vital. Under the high-temperature conditions of the PECVD process, graphite boats efficiently dissipate heat, maintaining a stable temperature environment essential for the uniform decomposition of gases. This thermal management ensures that the silicon and nitrogen ions combine accurately to form silicon nitride (SiNx) molecules, which then deposit uniformly on the wafer surface.
In summary, the superior electrical and thermal conductivity of graphite boats is fundamental to the success of PECVD processes. These properties not only facilitate the formation of glow discharge but also ensure the precise and uniform deposition of silicon nitride coatings, making graphite boats indispensable in the PECVD system.

Formation of Silicon Nitride Coating
The process of forming a silicon nitride (SiNx) coating on the wafer surface involves the decomposition of silane (SiH4) and ammonia (NH3) gases through a glow discharge. This discharge ionizes the gases, creating silicon (Si) and nitrogen (N) ions. These ions then combine to form SiNx molecules, which subsequently deposit onto the wafer.
Historically, silicon nitride was first produced by the direct reaction between elemental silicon (Si) and nitrogen (N2) or ammonia (NH3). This method, known as the direct nitriding method, involves high-purity silicon powder reacting with nitrogen or ammonia at elevated temperatures, typically around 1200-1400°C. The chemical equations for these reactions are:
- 3Si + 2N2 → Si3N4
- 3Si + 4NH3 → Si3N4 + 6H2
The formation of silicon nitride through these reactions results in a ceramic material with properties such as high strength, low density, and excellent high-temperature resistance. The structural unit of Si3N4 is the [SiN4]4- tetrahedron, where silicon atoms are located at the centers of the tetrahedrons, and nitrogen atoms occupy the vertex positions, creating a three-dimensional network structure.
In addition to the direct nitriding method, other techniques for producing silicon nitride include the carbothermal reduced silica method and various gas and liquid phase reaction methods. For instance, the carbothermal reduced silica method involves the reaction of silicon dioxide (SiO2) with carbon and nitrogen to form Si3N4:
- 3SiO2 + 6C + 2N2 → Si3N4 + 6CO
These diverse methods highlight the versatility and importance of silicon nitride in various industrial applications, from refractory materials to advanced ceramics used in mechanical processing, aerospace, and electronic circuits.
Chemical Stability and Durability
Graphite boats are renowned for their exceptional chemical stability, making them ideal for the harsh environments encountered in the PECVD process. These boats are engineered to withstand the corrosive effects of reaction gases and plasma, which are inherent to the PECVD system. The ability to resist chemical degradation is crucial, as any compromise in material integrity could lead to contamination or failure in the deposition process.
Moreover, graphite boats demonstrate remarkable stability under the high-temperature conditions that are a hallmark of the PECVD process. Operating temperatures often exceed 400°C, and graphite's thermal stability ensures that the boats maintain their structural and chemical integrity throughout these prolonged exposure periods. This durability is not only essential for the consistent performance of the PECVD system but also for the quality and uniformity of the silicon nitride coating formed on the wafer surface.
To further illustrate the importance of chemical stability and durability in the PECVD process, consider the following table:
| Property | Graphite Boats | Alternative Materials |
|---|---|---|
| Chemical Resistance | High | Variable |
| Thermal Stability | High | Low to Moderate |
| Structural Integrity | High | Variable |
| Coating Quality | High | Inconsistent |
This comparison underscores why graphite boats are the preferred choice in PECVD applications, ensuring not only the longevity of the equipment but also the reliability of the coating process.
Related Products
- Carbon Graphite Boat -Laboratory Tube Furnace with Cover
- High Purity Pure Graphite Crucible for Evaporation
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition