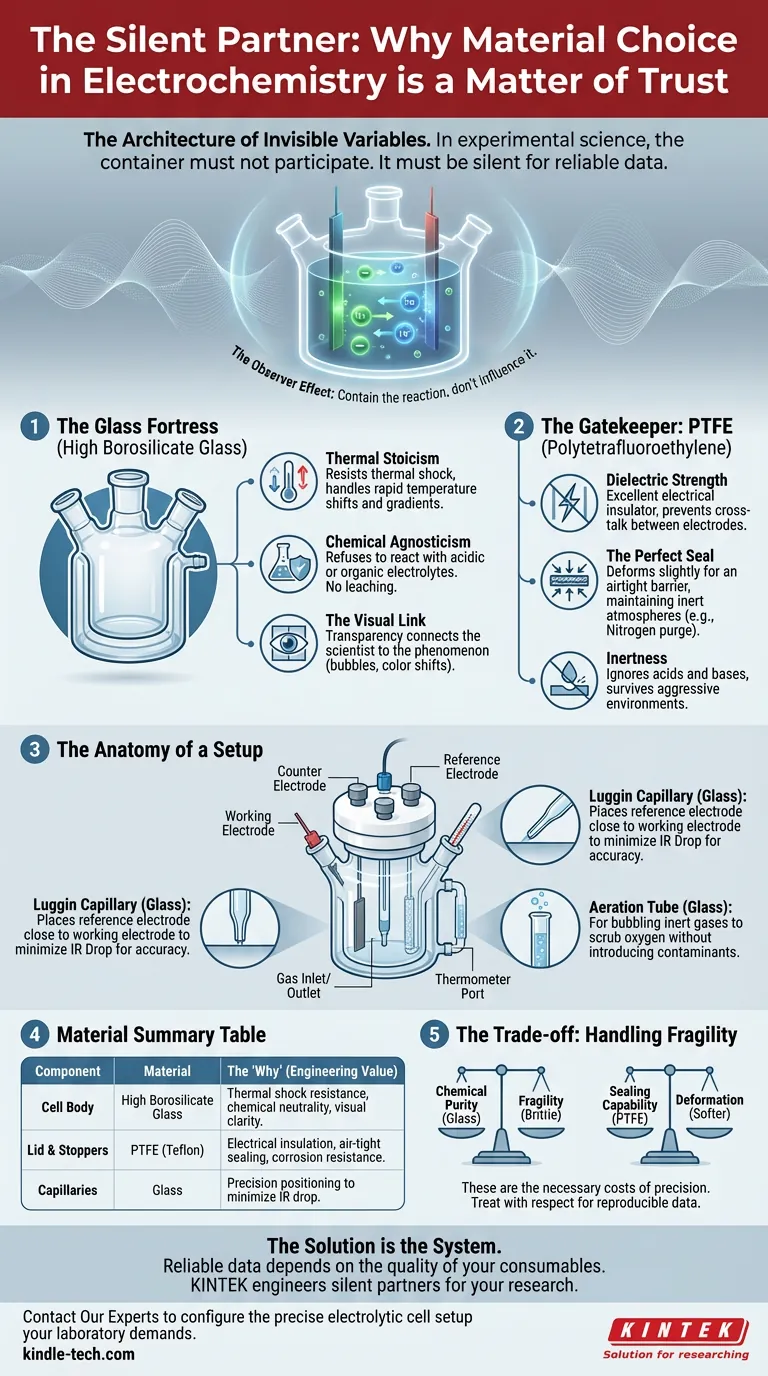
The Architecture of Invisible Variables
In experimental science, the vessel is never just a container. It is a variable.
If you read the history of failed experiments, you rarely find a dramatic explosion. You find data that "drifted." You find ghost peaks in a voltammogram. You find results that couldn't be replicated because the environment quietly interfered with the reaction.
This is the central tension of electrochemistry: The observer effect.
You need to contain a reaction to measure it, but the container itself must not participate. It must be invisible. It must be silent.
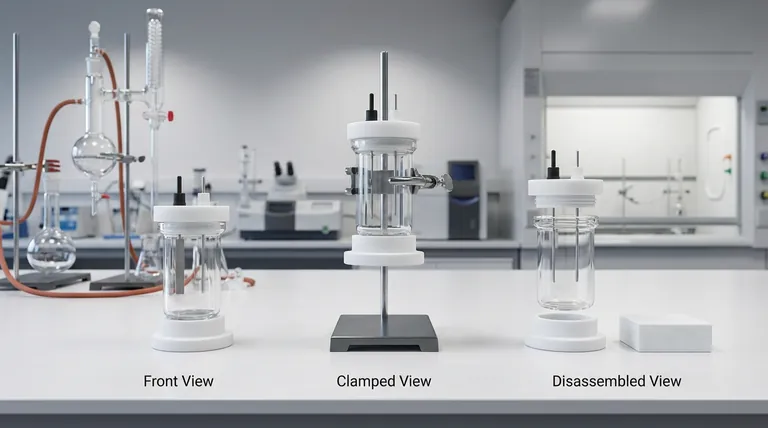
This is why the design of a five-port water bath electrolytic cell is not a matter of aesthetics. It is a matter of trust. The specific combination of High Borosilicate Glass and Polytetrafluoroethylene (PTFE) creates a sanctuary where the only chemistry happening is the chemistry you intended.
Here is the engineering logic behind that silence.
The Glass Fortress
The body of the cell is crafted from high borosilicate glass.
Standard glass is a chaotic material. It expands unevenly when heated and leaches ions into aggressive solutions. In sensitive electrochemical setups, standard glass is a saboteur.
High borosilicate glass is chosen for a specific psychological comfort: Certainty.
1. Thermal Stoicism
Electrolytic experiments often require precise temperature control via a water bath. This involves circulating hot or cold fluids around the cell jacket.
Borosilicate glass has an extremely low coefficient of thermal expansion. It resists "thermal shock." It allows you to shift temperatures rapidly without the catastrophic cracking that plagues cheaper materials. It holds the pressure of the thermal gradient without complaining.
2. Chemical Agnosticism
The glass must be chemically "agnostic." It cannot take a side.
Whether you are using acidic electrolytes or organic solvents, borosilicate glass refuses to react. This ensures that the ions you detect are the ones you added, not silicates leaching from the walls of your vessel.
3. The Visual Link
There is a romantic aspect to the transparency of glass. You need to see the gas bubbles forming on the electrode. You need to witness the color shift. Transparency connects the scientist to the phenomenon.
The Gatekeeper: PTFE
While the body is glass, the lid and stoppers are machined from Polytetrafluoroethylene (PTFE), commonly known as Teflon.
If the glass is the fortress, the PTFE lid is the gatekeeper.
In a five-port configuration, the lid is the most vulnerable point. It is where the outside world (oxygen, dust, humidity) tries to get in, and where the electrodes (high voltage) try to short out.
PTFE handles this chaos through three properties:
- Dielectric Strength: It is a phenomenal electrical insulator. It prevents cross-talk or short circuits between the working, counter, and reference electrodes, even when they are millimeters apart.
- The Perfect Seal: PTFE is slightly softer than metal or glass. When you tighten a stopper, it deforms microscopically to create an airtight seal, vital for maintaining inert atmospheres (like Nitrogen purging).
- Inertness: Like the glass body, PTFE ignores acids and bases. It survives environments that would dissolve other polymers.
The Anatomy of a Setup
The materials dictate the function. Because we use glass and PTFE, we can engineer specific tools that improve data fidelity.
- The Luggin Capillary: Made of glass, this narrow tube allows you to place the reference electrode extremely close to the working electrode. This reduces the "IR Drop" (voltage loss due to solution resistance), a common source of measurement error.
- The Aeration Tube: Also glass, allowing for the bubbling of inert gases to scrub oxygen from the system without introducing contaminants.
Material Summary
| Component | Material | The "Why" (Engineering Value) |
|---|---|---|
| Cell Body | High Borosilicate Glass | Thermal shock resistance, chemical neutrality, visual clarity. |
| Lid & Stoppers | PTFE (Teflon) | Electrical insulation, air-tight sealing, corrosion resistance. |
| Capillaries | Glass | Precision positioning to minimize IR drop. |
The Trade-off: Handling Fragility
There is no such thing as a perfect material. There are only trade-offs.
The price you pay for the chemical purity of borosilicate glass is fragility. It is brittle. A moment of carelessness when clamping the cell to a stand can shatter the jacket.
The price you pay for the sealing capability of PTFE is deformation. It is softer than metal. If you overtighten the electrodes, the ports can warp over time, compromising the seal.
These are not defects; they are the necessary costs of precision. Treat the equipment with the respect a surgical instrument deserves, and it will return the favor with reproducible data.
Designing Your Experiment
When you understand the materials, you stop fighting the equipment and start using it.
- For Accuracy: Trust the glass Luggin capillary to bridge the gap to your working electrode.
- For Air-Sensitivity: Trust the PTFE seal to hold your Nitrogen purge.
- For Versatility: Use the five ports. The glass transparency allows you to insert pH meters and thermometers without guessing their position.
The Solution is the System
In the end, you aren't just buying a glass jar. You are buying a controlled environment.
At KINTEK, we understand that the reliability of your data is capped by the quality of your consumables. We engineer our electrochemical cells to be the silent partners in your research—chemically inert, thermally stable, and meticulously designed.
Don't let your "invisible variables" ruin a perfect hypothesis.
Contact Our Experts today to configure the precise electrolytic cell setup your laboratory demands.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Related Articles
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells
- Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success