You’ve done everything by the book. Your samples are meticulously prepared, your process is documented, and you place the batch into the furnace, expecting clean, reliable data. But when the analysis comes back, it’s the same frustrating story: unexplained impurities, inconsistent material properties, and results that contradict your last run. You’re left wondering: what went wrong this time? It feels like an invisible saboteur is at work, wasting your valuable materials, your time, and your confidence.
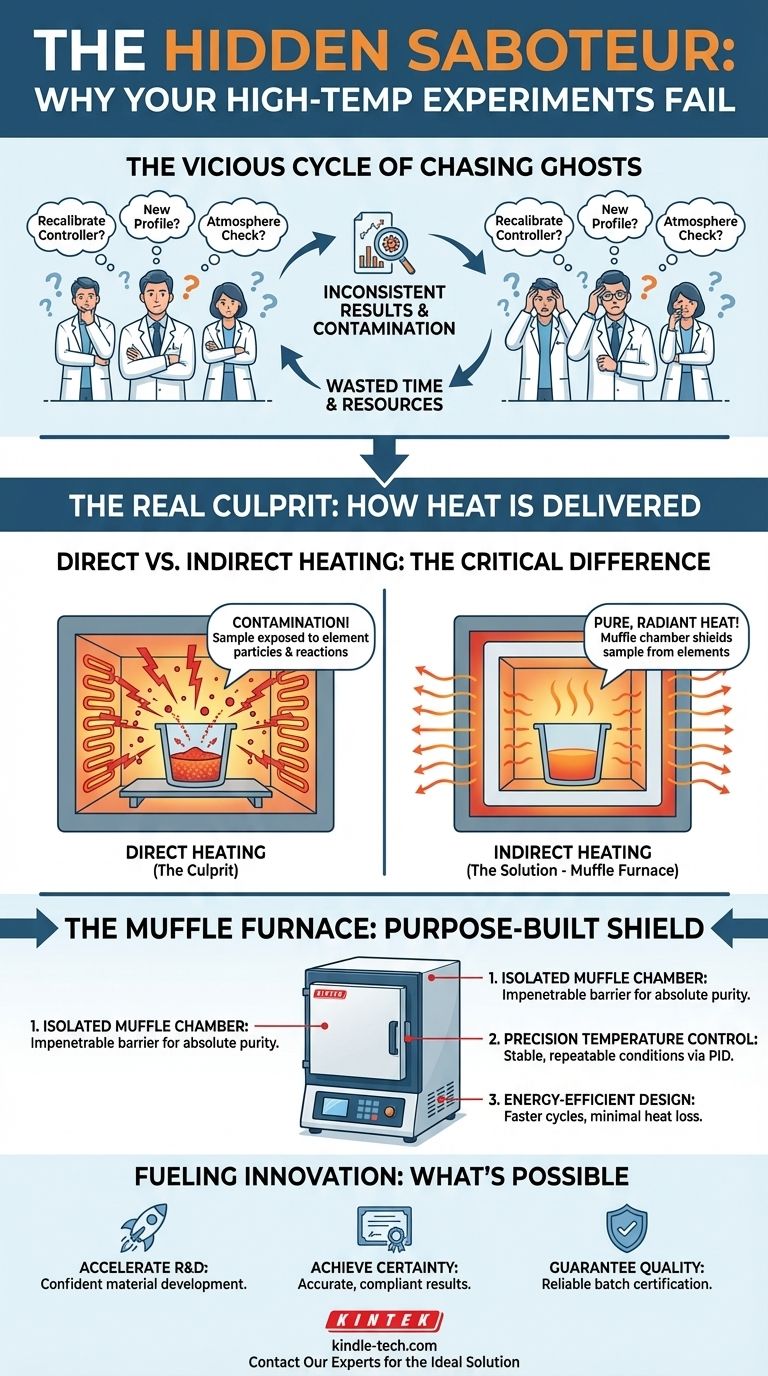
The Vicious Cycle of Chasing Ghosts

If this scenario sounds familiar, you are not alone. Across countless R&D, analytical, and quality control labs, teams find themselves trapped in a frustrating loop. The data is noisy, the results from ashing or sintering are unreliable, and no one can pinpoint the cause.
In response, a predictable pattern of "fixes" begins:
- "Let's recalibrate the temperature controller." You spend hours ensuring the temperature is accurate to a fraction of a degree, but the next batch is still inconsistent.
- "We need a different heating profile." Your team invests weeks testing various ramp rates and hold times, only to find the core problem of contamination or inconsistency persists.
- "Maybe it's the atmosphere?" You try purging the chamber, but some unknown variable continues to skew the outcome.
These efforts, while logical, often fail because they are focused on the symptoms, not the underlying disease. The commercial consequences are severe: critical projects stall, R&D budgets are consumed by repeat experiments, and unreliable quality control data puts product integrity at risk. You're not just losing experiments; you're losing momentum.
The Real Culprit: It’s Not the Heat, It’s How the Heat Is Delivered
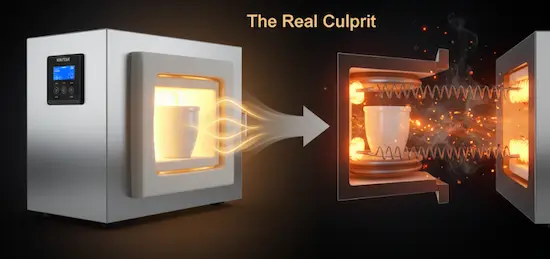
Here is the turning point. The problem in many of these failed experiments is not the amount or stability of the heat, but the fundamental way it is transferred to your sample. The issue is contamination from the heat source itself.
Direct vs. Indirect Heating: The One Concept You Need to Understand
Most people think of a furnace as a simple hot box. But how that box gets hot is critically important.
-
Direct Heating: In many furnaces, the sample shares the same space as the heating elements or, in fuel-fired units, the flame and combustion gases. At high temperatures, even electric heating elements can shed microscopic particles. These contaminants are free to land on or react with your sample, altering its chemical composition and ruining your results.
-
Indirect Heating: This is where the design of a muffle furnace becomes genius in its simplicity. Imagine cooking a stew in a sealed cast-iron pot that is placed inside a larger oven. The oven's heating elements heat the pot, and the pot’s hot walls then radiate heat to cook the stew perfectly. The food never touches the oven's heating elements.
A muffle furnace works on the exact same principle. The sample sits inside a separate, enclosed chamber (the "muffle"). The heating elements are on the outside of this chamber. They heat the chamber walls, which then radiate pure, uniform thermal energy onto the sample. This physical barrier makes it impossible for contaminants from the heat source to reach your material.
This is why the "common fixes" fail. No amount of temperature tweaking can prevent a heating element from contaminating a sample it shares a chamber with. You were treating a fever while ignoring the infection.
The Muffle Furnace: A Purpose-Built Shield for Your Samples

To truly solve this problem, you don't just need a furnace; you need a tool specifically engineered to prevent contamination. You need an environment where the only thing your sample is exposed to is controlled, radiant heat.
This is the philosophy behind KINTEK’s laboratory muffle furnaces. They are not just boxes that get hot; they are precision instruments designed to embody the principle of indirect heating, providing the ultimate shield for your work.
Here’s how our design directly solves the root problem:
- The Isolated Muffle Chamber: The core of our furnace is the high-purity ceramic muffle. This chamber acts as an impenetrable barrier, guaranteeing your sample—whether for ashing, sintering, or materials analysis—remains absolutely pure and free from external influence.
- Precision Temperature Control: Purity is only half the battle. Our furnaces integrate advanced PID controllers that work in concert with the insulated chamber to maintain exceptionally stable and uniform temperatures. This ensures your results are not only pure but also perfectly repeatable.
- Energy-Efficient Design: The heavy insulation required for stable, indirect heating also means minimal heat loss. This makes KINTEK muffle furnaces highly efficient, allowing for faster heat-up and cool-down cycles, which is perfect for busy labs running multiple batch processes.
From Fighting Fires to Fueling Innovation: What's Possible with Contamination-Free Heating
Once you eliminate the persistent, nagging problem of sample contamination, you’re no longer just avoiding bad results. You are unlocking new potential.
Think about what this means for your lab:
- Accelerate R&D: You can confidently develop and test sensitive, next-generation materials, knowing that the properties you measure are real, not artifacts of contamination. This drastically shortens the path from discovery to innovation.
- Achieve Analytical Certainty: For labs performing ashing for elemental analysis, you can achieve consistently accurate results that meet the most stringent regulatory or publication standards, eliminating the need for costly and time-consuming re-testing.
- Guarantee Product Quality: In a QC environment, you can reliably certify that every batch of material meets specification. This builds trust, reduces the risk of field failures, and protects your brand’s reputation.
By solving this fundamental problem, you move from a reactive state of troubleshooting to a proactive state of discovery and reliable production.
Your work is too important to be undermined by a fundamental equipment mismatch. If inconsistent results are holding back your projects, it’s time to address the root cause. Let's discuss how the right heating technology can ensure the integrity and accuracy of your most critical work. Contact Our Experts to explore the ideal solution for your laboratory's needs.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
Related Articles
- Why Your Ashing Tests Fail: The Hidden Difference Between Muffle and Ashing Furnaces
- Comprehensive Guide to Muffle Furnaces: Types, Uses, and Maintenance
- Muffle vs. Tube Furnace: How One Choice Prevents Costly Research Failures
- Muffle vs. Tube Furnace: How the Right Choice Prevents Catastrophic Lab Failure
- Why Your High-Temperature Experiments Fail: The Furnace Flaw Most Labs Overlook




















