Introduction to Zirconia Ceramic Sintering
Sintering Process Overview
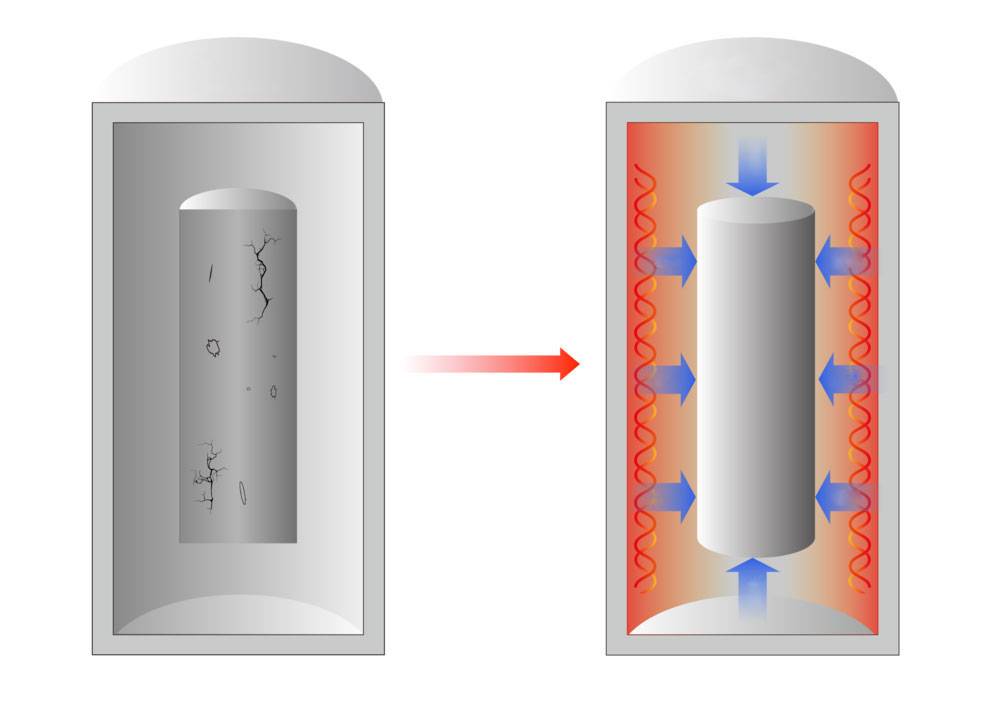
Before sintering, the ceramic blank is a composite of numerous individual solid particles, riddled with a significant number of pores. The porosity of these blanks typically ranges from 35% to 60%, translating to a relative density of 40% to 65%. This range is largely influenced by the intrinsic properties of the powder and the specific molding techniques employed.
Upon heating, the ceramic blank undergoes a series of intricate transformations. At elevated temperatures, the particles within the blank begin to migrate, facilitating the formation of necks between particles through processes such as diffusion and surface tension. As the sintering temperature is approached, typically set at 0.5 to 0.7 times the material's melting point, the blank starts to shrink. This shrinkage is accompanied by grain growth and a reduction in pore volume, ultimately leading to densification.
The sintering process can be broken down into several distinct stages:
- Formation and Compaction: The raw ceramic powder is initially formed into a specific shape, often through compaction techniques that ensure uniformity and minimize voids.
- Controlled Heating: The compacted material is then subjected to controlled heating within a sintering furnace. The temperature is meticulously regulated to promote particle bonding without inducing complete melting.
- Diffusion and Neck Formation: During heating, particle diffusion leads to the formation of necks, enhancing densification and reducing porosity.
- Cooling and Solidification: The final stage involves cooling the sintered product, allowing it to solidify into a cohesive, rigid structure.
This multi-stage process results in the transformation of the porous ceramic blank into a dense, polycrystalline material, retaining the original shape while significantly improving its mechanical properties.

Common Sintering Processes
Conventional Sintering
Conventional sintering remains the most prevalent method for producing ceramic materials, particularly zirconia ceramics. This technique involves the use of traditional electric furnaces to heat the prepared powder compact to the requisite temperature without applying external pressure. The simplicity of this method makes it accessible for use in both box and tube furnaces, though it necessitates a controlled atmosphere to ensure both safety and optimal results.
When pure ceramic materials prove challenging to sinter, sintering aids are often introduced. These additives facilitate the formation of low-melting point solid solutions, glass phases, or other liquid phases. This process aids in the rearrangement of particles and promotes viscous flow, ultimately leading to the production of dense, high-quality products. Notably, the incorporation of these sintering aids can also reduce the necessary sintering temperature, making the process more energy-efficient.
During conventional sintering, the ceramic material undergoes a heat treatment where the loose particles unite and bond, forming a solid piece. The temperature required for this process is always slightly lower than the melting point of the material, ensuring that the ceramic does not liquefy but instead achieves a dense, solid state. This method, while effective, does have limitations, such as the potential for uneven heating and the need for precise temperature control to avoid deformation or cracking.
Hot Pressing Sintering
Hot pressing sintering is a sophisticated process that integrates press molding with heat sintering in a single step, leveraging a specialized hot press. This method operates under high-temperature conditions, applying either single-phase or dual-phase pressure to the ceramic powder. The synergy between elevated temperatures and applied pressure significantly enhances the viscosity and plastic flow of the particles, facilitating the densification of the ceramic blanks. This densification process is instrumental in producing near-pore-free products, a significant advantage over other sintering techniques.
The benefits of hot pressing sintering are manifold. Firstly, the thermoplastic state of the powder during the process reduces deformation resistance, making it easier to achieve plastic flow and densification with minimal molding pressure. Secondly, the simultaneous application of heat and pressure promotes better contact, diffusion, and flow between powder particles, thereby lowering the sintering temperature and duration while suppressing grain growth. This results in a fine-grained structure that is close to theoretical density, with minimal porosity.
However, hot pressing sintering is not without its limitations. The process is constrained to producing products with relatively simple shapes due to the nature of the pressing and sintering mechanism. Additionally, the microstructure of the sintered material tends to be anisotropic, leading to performance anisotropy. This inherent characteristic restricts the scope of applications for hot pressing sintered products, particularly in scenarios requiring isotropic material properties.
In summary, while hot pressing sintering offers significant advantages in terms of densification, reduced sintering time, and grain size control, its applicability is limited by the complexity of shapes it can produce and the anisotropic properties of the final product.
Hot Isostatic Pressing
Hot isostatic pressing (HIP) is a sophisticated manufacturing process that leverages elevated temperature and isostatic gas pressure to enhance material properties. By employing inert gases like argon as the pressure medium, HIP places the product within a sealed container and applies uniform pressure from all directions under specific temperature and pressure conditions. This method effectively eliminates porosity and increases the density of materials such as metals, ceramics, polymers, and composites, thereby improving their mechanical properties and workability.
One of the key advantages of HIP is its ability to consolidate powders and facilitate diffusion bonding, often referred to as cladding. This process is particularly useful for eliminating microshrinkage in castings and is integral to the sintering process in powder metallurgy. Additionally, HIP is utilized for pressure-assisted brazing and the fabrication of metal matrix composites.
The mold material used in HIP is typically sheet metal, chosen for its high melting point to maintain structural integrity throughout the process. In certain specialized applications, ceramic molds are employed. The fluid used to pressurize the mold and form the part is usually an inert gas like argon, though a glass-like fluid is sometimes utilized. A common operational setting for HIP involves pressures of 15,000 lb/in² (100 MPa) at temperatures around 2000°F (1100°C).
While HIP technology demands high standards for sheath materials and techniques, it is particularly advantageous for producing ceramic products without the need for sheathing. Despite its limitations in handling products with complex shapes and its relatively lower production efficiency, HIP remains a crucial technique for achieving high-density, high-performance materials.
Microwave Sintering
Microwave sintering represents a distinct departure from conventional heating methods. This technique harnesses the dielectric loss properties of ceramic materials within a microwave electromagnetic field to elevate the material to the requisite sintering temperature, thereby facilitating the densification and consolidation of ceramics. During microwave sintering, the material actively absorbs microwaves, which are then converted into kinetic and potential energy within the molecular structure of the material. This conversion results in uniform heating across the entire material, minimizing internal temperature gradients and thermal stress. Consequently, microwave sintering enables rapid heating and sintering, facilitating low-temperature rapid densification and significantly enhancing the mechanical properties of ceramic materials.
The uniformity of microwave heating is a notable advantage, attributed to the high microwave transmittance of most ceramic materials. However, practical considerations such as heat dissipation from the sample surface can introduce challenges. Without appropriate insulation measures, the temperature differential between the interior and exterior of the heating body can become substantial, potentially leading to uneven sintering. Therefore, the design of an effective insulation layer is crucial to mitigate heat loss and ensure consistent sintering outcomes.

Moreover, microwave sintering is particularly advantageous for small loads, offering benefits such as faster heating rates, reduced energy consumption, and improvements in product properties. However, the process is typically limited to single compact sintering at a time, which can constrain overall productivity. Additionally, the penetration depth of microwaves is limited for materials with high conductivity and permeability, necessitating that the particle size of the powders be commensurate with the microwave penetration depth for optimal results. Despite these limitations, microwave sintering excels in maintaining fine grain sizes in bioceramics, underscoring its potential in specialized applications.
Common Problems in Sintering
Deformation
Zirconium oxide ceramics often undergo deformation during the sintering process, which can be attributed to several factors. Firstly, a wide distribution of powder particle sizes can lead to inconsistent shrinkage rates, causing the ceramic to deform. This inconsistency arises because smaller particles tend to shrink more rapidly than larger ones, leading to internal stresses within the ceramic body.
Secondly, the selection and addition of sintering aids or additives can significantly impact the deformation. If these additives are not chosen or added properly, they can create uneven chemical reactions or phase transitions, further exacerbating the deformation. For instance, the formation of low-melting point phases can cause localized melting, resulting in non-uniform shrinkage.
The inconsistency in ceramic shrinkage can also be traced to three primary reasons:
-
Uneven Furnace Temperature: If the temperature within the furnace is not uniform, the ceramic body will shrink inconsistently. Areas exposed to higher temperatures will shrink faster, leading to internal stresses and deformation.
-
Rapid Heating Speed: When the heating rate is too fast, a temperature gradient forms within the ceramic body. The surface of the ceramic heats up and shrinks more quickly than the core, creating a differential shrinkage that can cause warping or bending.
-
Density Gradient: During the molding process, factors such as pressure and the presence of fillers can create a density gradient within the ceramic body. This gradient results in varying shrinkage rates throughout the body, leading to deformation. For example, regions with higher density may shrink less compared to those with lower density, causing the ceramic to warp.
To mitigate these issues, careful control of powder characteristics, sintering aids, heating rates, and furnace temperature uniformity is essential. Additionally, optimizing the molding process to ensure uniform density distribution can significantly reduce the likelihood of deformation during sintering.

Cracking
The primary cause of cracking in sintered ceramic bodies is the presence of internal defects within the ceramic material, which is closely associated with the shrinkage characteristics of the body. Inconsistent shrinkage, a phenomenon often linked to sintering deformation, exacerbates this issue. When shrinkage varies across different regions of the ceramic body, any pre-existing defects such as voids or microcracks can act as initiation points for fractures. These defects, when subjected to stress due to inconsistent shrinkage, propagate rapidly, leading to cracks that can cause the entire body to fail.
To delve deeper into the mechanisms behind inconsistent shrinkage, several factors come into play:
-
Temperature Gradients: Uneven heating within the sintering furnace can result in inconsistent shrinkage rates. Areas exposed to higher temperatures will shrink more rapidly than those in cooler zones, leading to differential stresses.
-
Heating Rates: Rapid heating can create thermal gradients within the ceramic body. The surface layers heat up and shrink faster than the core, causing internal stresses that can lead to cracking if not managed properly.
-
Density Variations: During the molding process, variations in pressure and filler distribution can create density gradients within the green body. These density differences translate into uneven shrinkage during sintering, further contributing to the formation of cracks.
| Factor | Impact on Shrinkage | Potential Defects |
|---|---|---|
| Temperature Gradients | Creates differential shrinkage rates due to uneven heating. | Microcracks, voids |
| Heating Rates | Produces thermal gradients, causing surface-core shrinkage differences. | Surface cracks, delamination |
| Density Variations | Uneven densification leads to inconsistent shrinkage. | Density gradients, weak spots |
Understanding these factors is crucial for developing strategies to mitigate cracking during the sintering process, ensuring the production of high-quality ceramic materials.
Abnormal Grain Growth
Abnormal grain growth in zirconia ceramics is a critical issue that can significantly impact the material's final properties. This phenomenon occurs when certain grains in the ceramic matrix grow disproportionately larger than others, leading to a non-uniform microstructure. These oversized grains often contain numerous pores that are difficult to eliminate, as they are trapped within the grain boundaries. This pore entrapment hinders the material's ability to achieve higher densities, thereby degrading various material properties, particularly the mechanical ones such as fracture toughness and bending strength.
The primary causes of abnormal grain growth can be attributed to several factors:
-
Wide Particle Size Distribution: The initial powder used for ceramic fabrication often has a broad range of particle sizes. If the largest particles in the powder exceed twice the average grain size, it can lead to uneven grain growth during sintering.
-
Uneven Green Body Density: During the molding process, if the green body's density is not uniform—due to factors like powder agglomeration, variations in pressing pressure, or uneven distribution of additives—it can result in uneven densification during sintering.
-
Excessive Sintering Conditions: Overheating the ceramic material or maintaining it at high temperatures for extended periods can exacerbate grain growth. These conditions provide an environment where certain grains can grow abnormally large, further complicating pore elimination and densification.
Understanding these causes is crucial for developing strategies to mitigate abnormal grain growth, thereby improving the overall quality and performance of zirconia ceramics.
Related Products
- Precision Machined Yttria Stabilized Zirconia Ceramic Plate for Engineering Advanced Fine Ceramics
- Custom-Made Alumina Zirconia Special-Shaped Ceramic Plates for Engineering Advanced Fine Ceramics Processing
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
- Precision Machined Yttrium Stabilized Zirconia Ceramic Rod for Engineering Advanced Fine Ceramics
Related Articles
- An In-depth Study of Isostatic Presses: Types, Applications, and Advantages
- Unveiling the Exceptional Properties and Applications of Optical Quartz Plates
- Unlocking the Power of Optical Quartz Plates: Applications and Benefits
- Understanding the Process and Benefits of Zirconia Ceramic Cold Isostatic Pressing
- Isostatic Pressing of Ceramics: Process and Precision