In the pharmaceutical industry, freeze dryers are primarily used to remove water from sensitive drugs at low temperatures, a process called lyophilization. This technique is essential for preserving the stability, extending the shelf life, and maintaining the efficacy of products like vaccines, antibiotics, proteins, and other biological formulations that would be damaged by conventional heat-based drying methods.
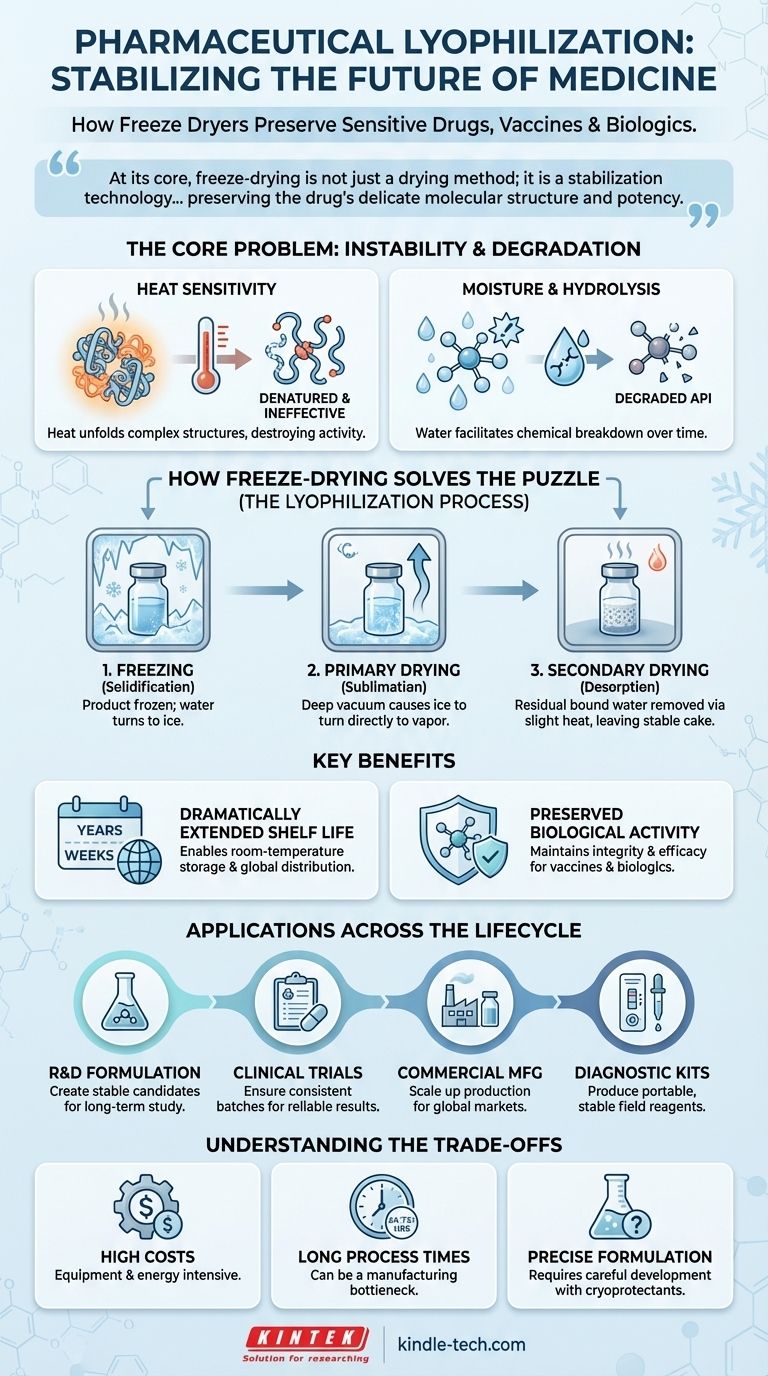
At its core, freeze-drying is not just a drying method; it is a stabilization technology. It solves the fundamental problem of drug degradation by removing water—the primary medium for chemical and biological instability—without using damaging heat, thereby preserving the drug's delicate molecular structure and potency.

The Core Problem: Instability in Modern Drugs
Many of the most advanced medicines, particularly biologics, are inherently fragile. Their complex structures are easily compromised by heat and moisture, rendering them ineffective or even unsafe.
The Challenge of Heat-Sensitive Biologics
Modern drugs like vaccines, enzymes, and therapeutic proteins are large, complex molecules. Their function depends entirely on their precise three-dimensional shape.
Applying heat, even at moderate levels, can cause these molecules to denature, or unfold, permanently destroying their biological activity. Freeze-drying bypasses this issue entirely by operating at very low temperatures.
The Threat of Moisture and Hydrolysis
Water is a universal solvent that facilitates chemical reactions, including those that degrade active pharmaceutical ingredients (APIs). This process, known as hydrolysis, can break down drug molecules over time.
By removing water to a residual level of less than 1%, freeze-drying effectively halts these degradation pathways, making long-term storage at room temperature possible for many products.
How Freeze-Drying Solves the Stability Puzzle
Lyophilization is a sophisticated, multi-stage process designed to gently remove water while keeping the product's structure intact. It is the gold standard for preserving fragile pharmaceutical products.
The Principle of Lyophilization
The process involves three main steps. First, the product is frozen to solidify the water. Next, during primary drying, the pressure is lowered to a deep vacuum, causing the frozen water to turn directly into vapor (sublimation).
Finally, during secondary drying, the temperature is slightly raised to remove any remaining, unfrozen water molecules. This leaves a dry, porous, and structurally sound "cake" that can be easily reconstituted with a sterile liquid before use.
Extending Shelf Life Dramatically
By removing the water that enables degradation, lyophilization can extend the shelf life of a drug from a few weeks or months to several years.
This stability is critical for building a reliable supply chain, reducing waste, and ensuring that medications are effective when they reach the patient, especially in regions without consistent refrigeration (the "cold chain").
Preserving Biological Activity
Because the entire process occurs at low temperatures and under a vacuum, the stress on the drug's molecular structure is minimized.
This preserves the integrity and efficacy of the original formulation, ensuring the drug delivers its intended therapeutic effect. For vaccines and biologics, this is non-negotiable.
Key Applications Across the Pharmaceutical Lifecycle
Freeze-drying is not a single-use technique; it is a platform technology applied at every stage of a drug's journey from the lab to the patient.
Research and Development (R&D)
In the early stages, scientists use laboratory freeze dryers to create stable formulations of new drug candidates. This allows for long-term study and characterization without the drug degrading between experiments.
Clinical Trials
For clinical studies, it is crucial that the investigational drug remains consistent and stable. Freeze-drying produces batches of the drug that maintain their potency over the duration of the trial, ensuring reliable and accurate results.
Commercial Manufacturing
On a large scale, freeze-drying is used to manufacture a wide array of commercial products, including injectable drugs, vaccines, antibiotics, and therapeutic proteins. It enables the creation of products that are stable enough for global distribution.
Diagnostic and Medical Kits
The technology is also used to produce portable and stable diagnostic kits. Reagents for medical tests can be freeze-dried, eliminating the need for refrigeration and allowing for their use in remote or field settings.
Understanding the Trade-offs
While powerful, lyophilization is not a universal solution. It involves significant technical and economic considerations that must be carefully weighed.
High Energy and Equipment Costs
Freeze dryers are complex machines that are expensive to purchase, install, and maintain. The process itself is also highly energy-intensive due to the long cycles and the need to maintain deep vacuums and low temperatures.
Long Process Times
A typical freeze-drying cycle can take anywhere from 24 to 72 hours or more. This long processing time can become a bottleneck in manufacturing, limiting throughput and increasing the cost per unit.
The Need for Precise Formulation
Not every drug solution can be successfully freeze-dried. The process requires a carefully developed formulation, often including cryoprotectants and other excipients, to protect the active ingredient from the stresses of freezing and drying.
How to Apply This to Your Project
Your decision to use freeze-drying should be directly tied to the specific stability requirements and economic realities of your product.
- If your primary focus is developing novel biologics or vaccines: Consider lyophilization an essential part of your formulation strategy to ensure the creation of a stable, distributable product from the outset.
- If your primary focus is extending the market life of an existing drug: Investigate freeze-drying as a reformulation method to improve stability, potentially eliminating cold-chain requirements and opening new markets.
- If your primary focus is operational efficiency in manufacturing: Carefully weigh the immense stability benefits of lyophilization against its high capital costs and long cycle times to determine if it is the most viable path for your product.
Ultimately, freeze-drying empowers the pharmaceutical industry to deliver fragile, life-saving medicines safely and effectively across the globe.
Summary Table:
| Key Application | Primary Benefit |
|---|---|
| Vaccines & Biologics | Preserves molecular structure & potency by avoiding heat damage. |
| Antibiotics & Injectables | Extends shelf life from months to years, enabling room-temperature storage. |
| R&D & Clinical Trials | Maintains drug consistency for reliable, long-term study results. |
| Diagnostic Kits | Creates stable, portable reagents for use in remote settings. |
Ready to ensure the stability and longevity of your pharmaceutical products? KINTEK specializes in providing high-quality laboratory freeze dryers and expert support for all your lyophilization needs. From R&D to commercial manufacturing, our equipment is designed to handle the delicate requirements of biologics, vaccines, and sensitive APIs. Contact our team today to discuss how we can help you develop a stable, effective formulation and optimize your freeze-drying process.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What is the function of Freeze-thaw Equipment in Au-(PNiPAAm/PVA) hydrogel? Achieve High-Speed Photothermal Actuation
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- Why is a freeze dryer preferred over thermal drying for Fe-ZTA cermets? Ensure Pure, Homogeneous Slurry Processing
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis



















