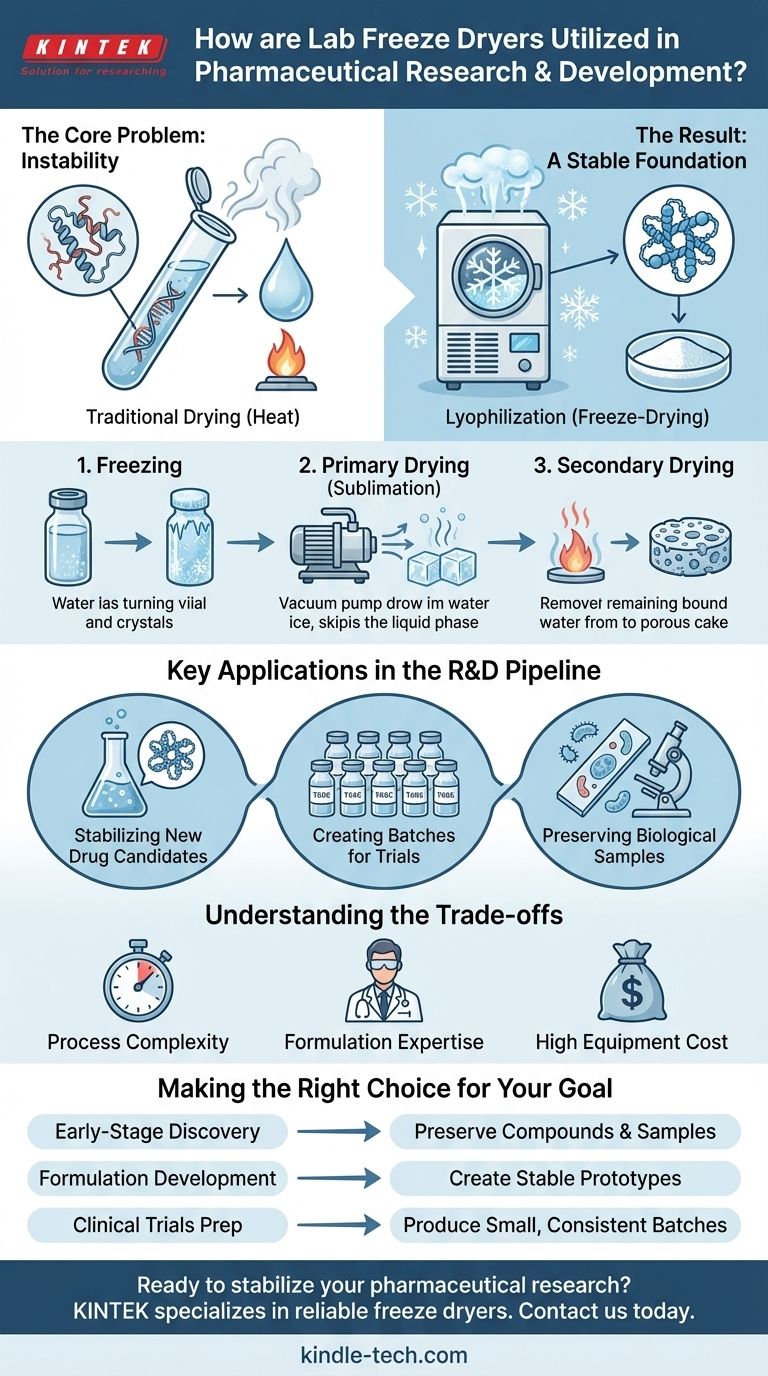
In pharmaceutical research and development, lab freeze dryers are critical for stabilizing delicate new drug candidates and preserving biological samples. Using a low-temperature dehydration process called lyophilization, these machines remove water from heat-sensitive compounds without damaging their molecular structure or biological activity. This enables researchers to produce small, stable batches of investigational drugs for preclinical testing and clinical trials.
The core challenge in modern drug development is managing the inherent fragility of complex molecules like proteins, vaccines, and certain APIs. Freeze-drying solves this by preserving a compound's integrity, making it stable enough for storage, transport, and testing, which underpins the reliability of the entire R&D pipeline.

The Core Problem: Instability of Modern Medicines
Why Traditional Drying Fails
Many of today's most promising drugs, especially biologics like proteins and vaccines, are extremely sensitive to heat.
Traditional drying methods, which use heat to evaporate water, would cause these complex molecules to denature—unfold and lose their specific three-dimensional shape. This structural change is irreversible and renders the drug biologically inactive and therefore useless.
How Freeze-Drying Works
Freeze-drying, or lyophilization, bypasses the destructive effects of heat by removing water at low temperatures and low pressure.
The process involves three main stages:
- Freezing: The material is frozen solid, locking water molecules in place as ice.
- Primary Drying (Sublimation): The pressure is lowered to a near-vacuum, and a small amount of energy is added. This causes the ice to turn directly into water vapor, skipping the liquid phase entirely.
- Secondary Drying: Any remaining unfrozen water molecules are gently removed from the material.
The Result: A Stable Foundation for Research
The final product is a dry, porous, and lightweight solid often called a "cake." This lyophilized material is chemically stable at room temperature and can be stored for long periods.
Crucially, it retains the drug's original biological activity and can be quickly and easily reconstituted with a sterile liquid before use.
Key Applications in the R&D Pipeline
Stabilizing New Drug Candidates
During early-stage research, scientists synthesize or discover new Active Pharmaceutical Ingredients (APIs). Freeze-drying is used to preserve these novel compounds, whether they are small molecules or large biologics, ensuring they remain stable for further characterization and testing.
Creating Batches for Preclinical and Clinical Trials
Once a promising drug candidate is identified, it must be formulated into a usable product for testing. Lab-scale freeze dryers are used to produce the small, consistent batches required for preclinical animal studies and early-phase human clinical trials.
This ensures that the exact same formulation is being tested at every stage, which is vital for generating reliable and valid data.
Preserving Biological Samples for Analysis
Pharmaceutical R&D relies on the accurate analysis of biological samples like enzymes, cell cultures, and tissues. Freeze-drying preserves these materials in a near-native state.
This prevents degradation and ensures that experimental results are accurate and reproducible, which is fundamental to making sound scientific and business decisions.
Understanding the Trade-offs
Process Complexity and Time
Lyophilization is a highly technical, slow, and energy-intensive process. A single cycle can take anywhere from several hours to multiple days, making it significantly more time-consuming than other drying methods.
The Need for Formulation Expertise
Not all drug solutions can be successfully freeze-dried. The process requires careful formulation development to include excipients (stabilizing agents) that protect the API during freezing and drying stresses, ensuring the final product is both stable and effective.
High Equipment Cost
Freeze-drying equipment is a significant capital investment. The technology required to maintain very low temperatures and pressures is complex and expensive, both to purchase and to operate.
Making the Right Choice for Your Goal
By understanding its specific applications, you can strategically leverage lyophilization to advance your project.
- If your primary focus is early-stage drug discovery: Use freeze-drying to preserve the stability of new compounds and biological samples, ensuring your foundational research is accurate.
- If your primary focus is formulation development: Leverage lyophilization to create and test stable prototypes of your drug product for shelf-life studies and preclinical evaluation.
- If your primary focus is preparing for clinical trials: Employ lab-scale freeze dryers to produce the small, consistent batches of your investigational drug needed for Phase I studies.
Ultimately, mastering freeze-drying transforms a fragile discovery into a viable therapeutic candidate ready for further development.
Summary Table:
| Application | Purpose | Key Benefit |
|---|---|---|
| Stabilizing New Drug Candidates | Preserve novel APIs and biologics | Maintains molecular integrity and biological activity |
| Batch Production for Trials | Create consistent preclinical/clinical batches | Ensures reliable, reproducible testing data |
| Preserving Biological Samples | Protect enzymes, cell cultures, and tissues | Enables accurate, reproducible analysis |
Ready to stabilize your pharmaceutical research with precision lyophilization? KINTEK specializes in lab freeze dryers and consumables, providing the reliable equipment you need to preserve delicate drug candidates and biological samples. Our expertise supports your entire R&D pipeline—from early discovery to clinical trials—ensuring your compounds remain stable and effective. Contact us today to discuss how our solutions can enhance your lab's efficiency and accelerate your drug development process!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How is freeze drying used in biological applications? Preserving Vital Biomaterials for Research & Pharma
- What is the purpose of laboratory freeze drying? Preserve Sensitive Drugs & Biologics for Stability
- How does capacity affect the price of a lab freeze dryer? Find the Right Fit for Your Lab
- What are the steps to use a laboratory freeze dryer? Master Lyophilization for Superior Sample Preservation
- How does freeze drying benefit dairy products? Unlock Premium Quality and Shelf Stability



















