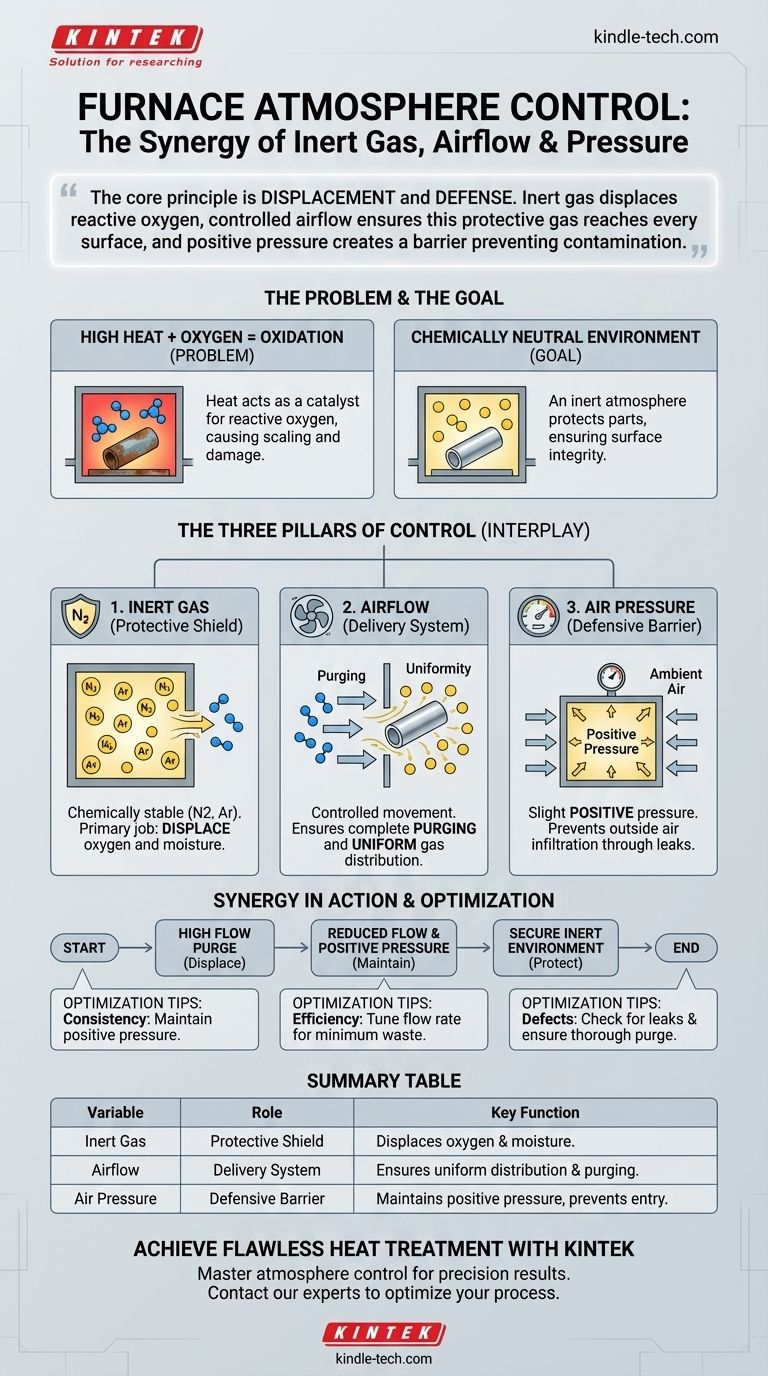
In essence, inert gas technology, airflow, and air pressure are the three critical levers for controlling a furnace's internal atmosphere. They work together to create and maintain a chemically non-reactive environment, which is essential for protecting parts from damage like oxidation during high-temperature processing.
The core principle is displacement and defense. The inert gas displaces reactive oxygen, while controlled airflow ensures this protective gas reaches every surface, and positive pressure creates a barrier that prevents outside air from contaminating the process.

The Core Problem: Why Atmosphere Control is Critical
At room temperature, most metals are relatively stable in the air. Introducing the intense heat of a furnace, however, dramatically changes the rules of chemistry.
High Temperatures as a Catalyst
Heat acts as a powerful catalyst for chemical reactions. The energy it provides allows atoms to overcome their natural stability and react with their surroundings much more quickly.
The Enemy: Oxidation and Contamination
The most common enemy in a furnace is oxygen. At high temperatures, oxygen will aggressively bond with most metals, creating oxides—a process we see as discoloration, scaling, or rust. This oxidation can ruin a part's surface finish, dimensional accuracy, and structural integrity.
The Goal: A Chemically Neutral Environment
The primary goal of furnace atmosphere control is to create a chemically neutral, or inert, environment. This is an atmosphere that will not react with the parts being processed, regardless of the high temperatures involved.
The Three Pillars of Furnace Atmosphere
Achieving a stable, inert atmosphere relies on the precise interplay of three distinct but interconnected factors.
Pillar 1: Inert Gas (The Protective Shield)
An inert gas, such as nitrogen or argon, is chemically stable and does not readily react with other elements.
Its primary job is to displace the oxygen and moisture from the furnace chamber. By flooding the space with a non-reactive gas, you effectively remove the fuel for unwanted chemical reactions.
Pillar 2: Airflow (The Delivery System)
Airflow refers to the controlled movement and flow rate of the inert gas into and through the furnace. It is not about turbulence, but about methodical replacement.
Properly managed airflow ensures two things:
- Purging: It completely flushes out the ambient, oxygen-rich air before the heating process begins.
- Uniformity: It maintains an even distribution of the inert gas throughout the chamber, preventing "dead spots" where reactive gases could become trapped.
Pillar 3: Air Pressure (The Defensive Barrier)
This involves maintaining a slight positive pressure inside the furnace relative to the atmospheric pressure outside.
This is a critical defensive measure. If any small leaks exist in the furnace seals, the higher internal pressure ensures that inert gas flows out rather than ambient air seeping in. This barrier is fundamental to preventing contamination throughout the heating cycle.
Understanding the Trade-offs and Pitfalls
Balancing these three pillars is key to both quality and efficiency. An imbalance in one area compromises the entire system.
The Cost of Imbalance
If the pressure is too low (or negative), outside air will be drawn into the chamber, causing immediate oxidation and ruining the parts.
If the airflow is too low, the initial purge may be incomplete, leaving pockets of oxygen that cause isolated defects.
If the airflow is too high, you are simply wasting expensive inert gas and money. Excessive flow can also create unwanted temperature variations within the furnace.
Synergy in Action
A typical process demonstrates their interplay perfectly. First, a high flow rate of inert gas purges the chamber. Once purged, the flow is reduced to a lower level, and positive pressure is established and maintained for the duration of the heating and cooling cycle, guaranteeing a secure, inert environment.
Optimizing Your Furnace Process
Applying these principles allows you to move from simply heating parts to precisely engineering their final properties.
- If your primary focus is process consistency: Ensure you are always maintaining a slight positive pressure; this is your single best defense against random contamination events.
- If your primary focus is cost efficiency: Carefully tune your inert gas flow rate to find the minimum level required to effectively purge the chamber and maintain pressure without excessive waste.
- If you are experiencing defects like discoloration: Your first step should be to check for leaks and then verify that your initial purge cycle is long and thorough enough to displace all contaminants.
Mastering the balance of inert gas, airflow, and pressure transforms a furnace from a simple oven into a precision instrument for material processing.
Summary Table:
| Variable | Role in the Furnace | Key Function |
|---|---|---|
| Inert Gas | Protective Shield | Displaces oxygen and moisture to prevent chemical reactions. |
| Airflow | Delivery System | Ensures uniform gas distribution and complete purging of contaminants. |
| Air Pressure | Defensive Barrier | Maintains positive pressure to prevent outside air from entering. |
Achieve flawless heat treatment results with KINTEK.
Mastering furnace atmosphere control is critical for protecting your materials from oxidation and contamination. Whether your goal is ultimate process consistency, cost efficiency, or eliminating defects, the right equipment is key.
KINTEK specializes in precision lab furnaces and expert support for all your laboratory needs. We provide the technology and knowledge to help you optimize inert gas usage, airflow, and pressure for perfect results every time.
Ready to transform your furnace into a precision instrument? Contact our experts today to discuss your specific application and discover the KINTEK solution for you.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What gases are used in inert atmospheres? Choose the Right Gas for Non-Reactive Environments
- What is an inert condition? A Guide to Preventing Fires and Explosions
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety
- How do you make an inert atmosphere? Master Safe, Pure Processes with Inerting
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes



















