Reactivating carbon is fundamentally a process of thermal purification. It involves heating spent activated carbon to very high temperatures in a controlled, oxygen-free environment. This process uses steam as a selective oxidizing agent to burn off the organic contaminants that have been adsorbed onto the carbon, thereby restoring its porous structure and adsorptive capacity.
The core challenge of reactivation is not simply heating the carbon, but precisely controlling temperature and atmospheric conditions. The goal is to destroy the adsorbed contaminants without damaging the carbon's vast internal network of pores, which is the very source of its effectiveness.

The Goal of Reactivation: Restoring Porosity
To understand reactivation, you must first understand why carbon becomes "spent." The process is about reversing the mechanism that makes it work in the first place.
What is "Spent" Carbon?
Activated carbon works because it has an incredibly high internal surface area, composed of millions of microscopic pores. When it is used to purify water or air, organic molecules (contaminants) get trapped within this pore network in a process called adsorption.
"Spent" carbon is simply carbon whose pores have become saturated or clogged with these adsorbed contaminants, rendering it unable to capture more.
Restoring the Adsorption Capacity
Reactivation is a destructive process designed to empty these clogged pores. By applying extreme heat in a controlled atmosphere, the adsorbed organic compounds are broken down and vaporized, freeing up the pore structure to be used again.
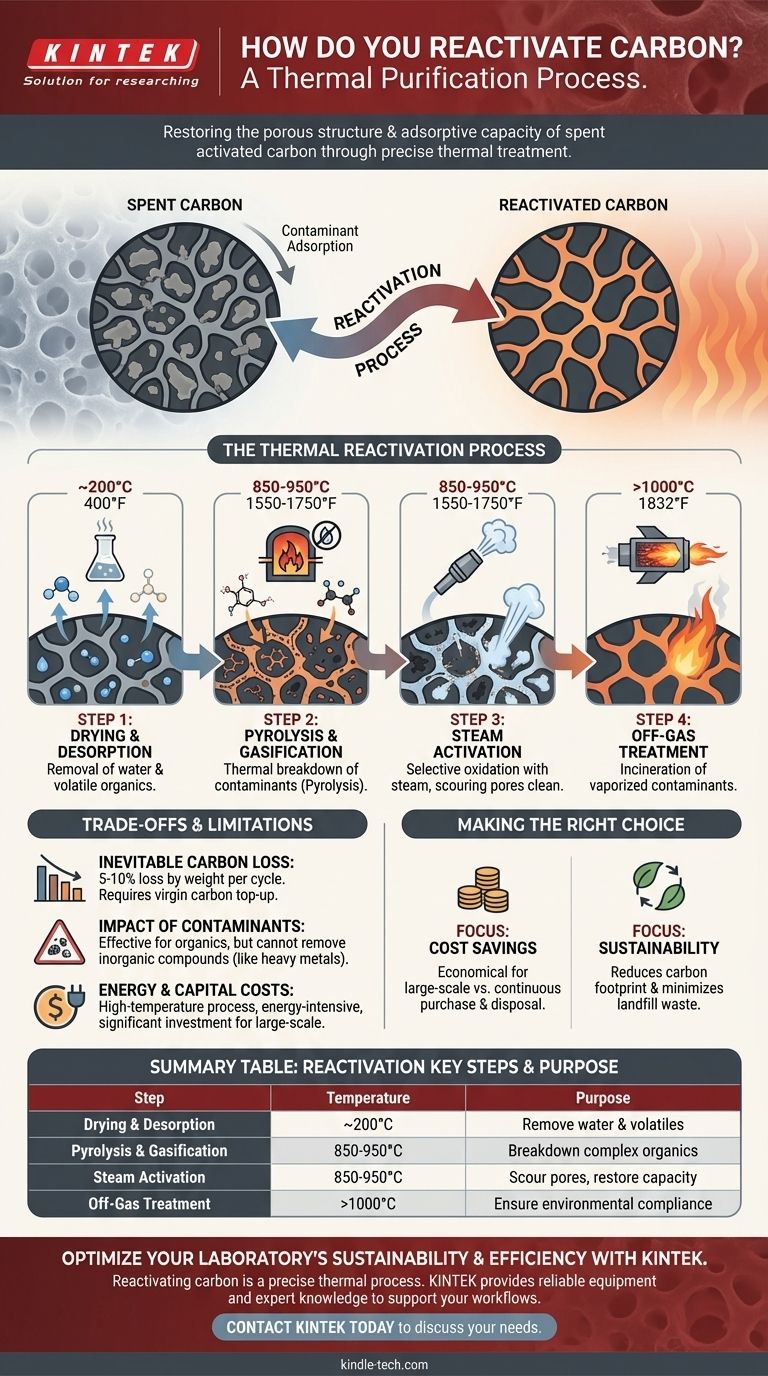
The Thermal Reactivation Process
While specifics can vary, the industrial standard for reactivation follows a clear, multi-stage thermal sequence, typically performed in a rotary kiln or multiple hearth furnace.
Step 1: Drying and Desorption
The spent carbon is first heated to around 200°C (400°F). This initial phase drives off any residual water and desorbs the most volatile organic compounds that were captured by the carbon.
Step 2: Pyrolysis and Gasification
This is the core of the reactivation. The carbon is heated further to high temperatures, typically between 850-950°C (1550-1750°F), in an oxygen-starved environment.
At these temperatures, the larger, less volatile organic contaminants are broken down (pyrolyzed) into smaller molecules and elemental carbon, or char.
Step 3: The Role of Steam
Injecting steam at this high temperature is the critical step. The steam acts as a selective oxidizing agent, initiating a gasification reaction.
It reacts with the pyrolyzed contaminant char, converting it into carbon monoxide and hydrogen gas. This process effectively scours the internal pore network, clearing it out without significantly damaging the base activated carbon structure.
Step 4: Off-Gas Treatment
The gases released from the furnace—composed of vaporized contaminants and the byproducts of gasification—are extremely hazardous. These off-gases must be routed to a secondary combustion chamber, or afterburner, where they are incinerated at temperatures exceeding 1000°C (1832°F) to ensure complete destruction and compliance with environmental regulations.
Understanding the Trade-offs and Limitations
Reactivation is a powerful tool for sustainability and cost management, but it is not a perfect or limitless process. It comes with clear trade-offs that must be considered.
Inevitable Carbon Loss
Each reactivation cycle is aggressive. A portion of the original activated carbon, typically 5-10% by weight, is inevitably lost during the process. This material must be replaced with virgin carbon to maintain the total volume.
Impact of Contaminant Type
Thermal reactivation is highly effective for organic contaminants. However, it cannot remove inorganic compounds like heavy metals. These materials can accumulate in the carbon over multiple cycles, potentially poisoning its effectiveness or damaging the furnace equipment itself.
Energy and Capital Costs
Reactivation is an energy-intensive process that requires significant capital investment. The high temperatures and sophisticated pollution control equipment (the afterburner and scrubbers) make it viable primarily for larger-scale industrial operations that handle substantial quantities of spent carbon.
Making the Right Choice for Your Goal
Deciding whether to reactivate carbon or purchase virgin material depends entirely on your operational priorities.
- If your primary focus is cost savings in large-scale operations: Reactivation is often more economical than continually purchasing and disposing of virgin carbon, despite the initial investment.
- If your primary focus is environmental sustainability: Reactivating carbon dramatically reduces the carbon footprint associated with producing new carbon and minimizes landfill waste.
- If you are dealing with unknown or mixed contaminants: You must first analyze the spent carbon to ensure the impurities are thermally destructible and won't harm the carbon or the reactivation facility.
Ultimately, successful carbon reactivation is a precise engineering process that balances the complete destruction of contaminants with the careful preservation of the carbon's essential porous structure.
Summary Table:
| Reactivation Step | Key Process | Temperature Range | Purpose |
|---|---|---|---|
| Drying & Desorption | Removal of water & volatile organics | ~200°C (400°F) | Prepare carbon for high-temperature treatment |
| Pyrolysis & Gasification | Thermal breakdown of contaminants | 850-950°C (1550-1750°F) | Destroy complex organic molecules trapped in pores |
| Steam Activation | Selective oxidation with steam | 850-950°C (1550-1750°F) | Scour pores clean, restoring adsorption capacity |
| Off-Gas Treatment | Incineration of vaporized contaminants | >1000°C (1832°F) | Ensure environmental compliance and safety |
Optimize your laboratory's sustainability and efficiency with KINTEK.
Reactivating carbon is a precise thermal process that requires reliable equipment and expert knowledge. Whether you are looking to reduce operational costs or minimize your environmental footprint, KINTEK's specialized lab equipment and consumables are designed to support your reactivation and purification workflows.
We provide the durable, high-performance tools your laboratory needs to handle materials like activated carbon effectively. Let our experts help you select the right equipment for your specific application.
Contact KINTEK today to discuss how our solutions can enhance your lab's capabilities and contribute to your sustainability goals.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the purpose of the rotary kiln? A Guide to Continuous High-Temperature Processing
- What role does a high-temperature rotary kiln play in the production of cement clinker? Mastering Sintering Efficiency
- What are disadvantages of pyrolysis process? Key Challenges in Energy, Cost, and Product Stability
- What are the parameters of a rotary kiln? Mastering Control for Optimal Process Results
- What are the basics of a rotary kiln? A Guide to Industrial-Scale Material Processing
- What are the parts of a carbon regeneration kiln? A Guide to Its Core Components and Function
- What are the advantages of catalytic pyrolysis? Produce High-Value Biofuels from Biomass
- What is the technique of pyrolysis? A Guide to Thermal Decomposition Without Oxygen



















