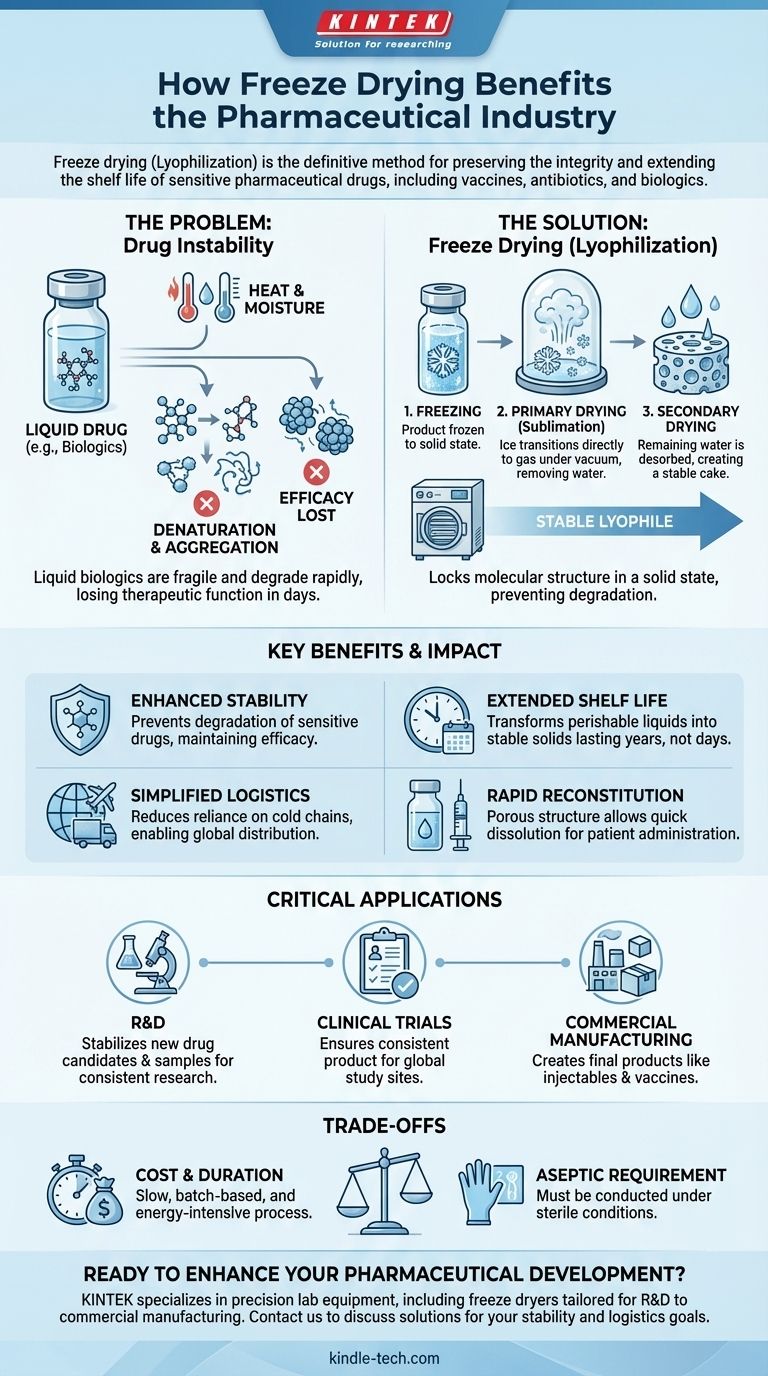
In the pharmaceutical industry, freeze drying is the definitive method for preserving the integrity and extending the shelf life of sensitive drugs. This process, also known as lyophilization, is critical for manufacturing stable vaccines, antibiotics, proteins, and other biologic-based medicines that would otherwise degrade rapidly, ensuring they remain effective from production to patient administration.
The core challenge for many advanced medications is their inherent instability in liquid form. Freeze drying solves this by removing water at low temperatures, effectively pausing degradation and locking the drug's molecular structure into a stable, solid state for long-term storage and transport.

The Fundamental Problem: Drug Instability
Many of modern medicine's most powerful treatments are also its most fragile. Biologics, in particular, present a significant preservation challenge.
Why Heat and Moisture Are Enemies
Active Pharmaceutical Ingredients (APIs), especially large molecules like proteins and enzymes, are highly susceptible to chemical and physical degradation.
When dissolved in water, these molecules can react, clump together (aggregate), or break down, losing their therapeutic function. Heat dramatically accelerates this process.
The Fragility of Biologics
Vaccines, therapeutic proteins, and enzymes derive their function from a precise, complex three-dimensional shape.
Even moderate heat can cause these molecules to "denature," or unfold, permanently destroying their efficacy. This makes conventional heat-based drying methods entirely unsuitable.
How Freeze Drying Preserves Efficacy
Lyophilization is an elegant solution that circumvents the damaging effects of heat and liquid-state degradation. It is a three-step process involving freezing, a primary drying phase under vacuum, and a secondary drying phase.
The Principle of Sublimation
Freeze drying's core principle is sublimation—the transition of a substance directly from a solid (ice) to a gas (water vapor) without passing through a liquid phase.
By freezing the product and then applying a deep vacuum, the water molecules are gently removed. This avoids the structural stress and chemical reactions that occur in a liquid state.
Locking in the Molecular Structure
The process effectively creates a stable "scaffold" of the drug molecules, locking them in their correct orientation. The final product is a dry, porous cake known as a lyophile.
This solid-state form prevents molecular mobility, halting the degradation pathways that render liquid formulations useless over time.
Extending Shelf Life from Days to Years
A liquid biologic might be stable for only days or weeks, even under refrigeration.
Once properly lyophilized, the same product can remain stable for several years at room or refrigerated temperatures, a critical factor for managing inventory and ensuring global access.
Critical Applications Across the Drug Lifecycle
Freeze drying is not just a manufacturing step; it is an enabling technology used from the earliest stages of research to the final commercial product.
In Research and Development (R&D)
Scientists use laboratory-scale freeze dryers to stabilize new drug candidates and precious biological samples. This ensures that research results are consistent and that novel compounds can be studied over time without degrading.
For Clinical Trials
Producing stable investigational drugs is essential for clinical trials. Lyophilization allows a consistent product to be manufactured and shipped to trial sites around the world, ensuring all participants receive the same dose and formulation.
In Commercial Manufacturing
On a large scale, freeze drying is used to create final commercial products. This includes a vast range of injectables, vaccines, and even portable diagnostic kits that require stable enzymes to function correctly in the field.
The Practical Advantages of Lyophilized Products
Beyond stability, freeze-dried drugs offer significant logistical and clinical benefits.
Simplified Storage and Transport
Freeze-dried powders are lightweight and far less sensitive to temperature fluctuations than their liquid counterparts. This dramatically reduces the reliance on a stringent and expensive "cold chain" for shipping and storage.
This benefit is particularly crucial for distributing vaccines and medicines to remote or developing regions where consistent refrigeration is not guaranteed.
Rapid Reconstitution for Patient Use
The porous cake structure created by lyophilization allows the product to be reconstituted rapidly.
Before administration, a healthcare professional can quickly dissolve the powder with a sterile liquid (like sterile water), ensuring the patient receives the drug in its fully active form.
Understanding the Trade-offs
While indispensable, lyophilization is a complex and resource-intensive process with specific challenges.
Process Cost and Duration
Freeze drying is a slow, batch-based process that can take several days to complete. The specialized equipment and high energy consumption make it one of the most expensive unit operations in pharmaceutical manufacturing.
Requirement for Aseptic Processing
Because many lyophilized drugs are injectables, the entire process must be conducted under sterile (aseptic) conditions to prevent microbial contamination. This adds significant complexity and cost to facility design and operation.
Formulation Development
Not every drug formulation is suitable for freeze drying. Significant development work is required to create a recipe with the right excipients (stabilizing ingredients) that can protect the API during the freezing and drying stresses and result in a stable, elegant final product.
Applying This to Your Goal
Your approach to lyophilization depends entirely on your specific objective within the pharmaceutical value chain.
- If your primary focus is long-term stability for a biologic: Freeze drying is the industry standard and often the only viable path to creating a product with a multi-year shelf life.
- If your primary focus is simplifying global logistics: Lyophilization creates a lightweight, temperature-tolerant product that drastically reduces cold-chain costs and complexity.
- If your primary focus is early-stage drug discovery: Use lab-scale freeze drying to preserve the integrity of new compounds and biological samples, ensuring consistency in your research.
- If your primary focus is creating user-friendly diagnostics: Incorporating lyophilized reagents is the key to producing stable, portable kits that deliver accurate results in the field.
Ultimately, freeze drying is a foundational technology that makes many of modern medicine's most advanced therapies possible.
Summary Table:
| Key Benefit | Impact on Pharmaceutical Products |
|---|---|
| Enhanced Stability | Prevents degradation of proteins, vaccines, and biologics, maintaining efficacy. |
| Extended Shelf Life | Transforms perishable liquids into stable solids lasting years, not days. |
| Simplified Logistics | Reduces reliance on cold chains, enabling global distribution to remote areas. |
| Rapid Reconstitution | Porous cake structure allows quick dissolution for patient administration. |
Ready to enhance your pharmaceutical development with reliable freeze-drying solutions? KINTEK specializes in precision lab equipment, including freeze dryers tailored for R&D, clinical trials, and commercial manufacturing. Whether you're stabilizing sensitive biologics, developing vaccines, or creating portable diagnostics, our expertise ensures your products maintain integrity from lab to patient. Contact us today to discuss how our solutions can support your stability and logistics goals!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Liquid Nitrogen Cryogenic Grinder Mill Cryomill Airflow Ultrafine Pulverizer
- Manual Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
People Also Ask
- How does an autoclave ensure the reliability of experimental results? Achieving a Sterile Baseline for Lab Research
- How is an autoclave utilized in antimicrobial experiments? Ensure Precise Nanoparticle Research Integrity
- How should solid materials in bags be prepared for decontamination? Master Steam Penetration for Safe Sterilization
- What is the primary purpose of an autoclave in the preparation of media for the biological leaching of uranium?
- Why is the prevention of air entrapment critical for the autoclave sterilization process? Ensure 100% Sterility Today



















