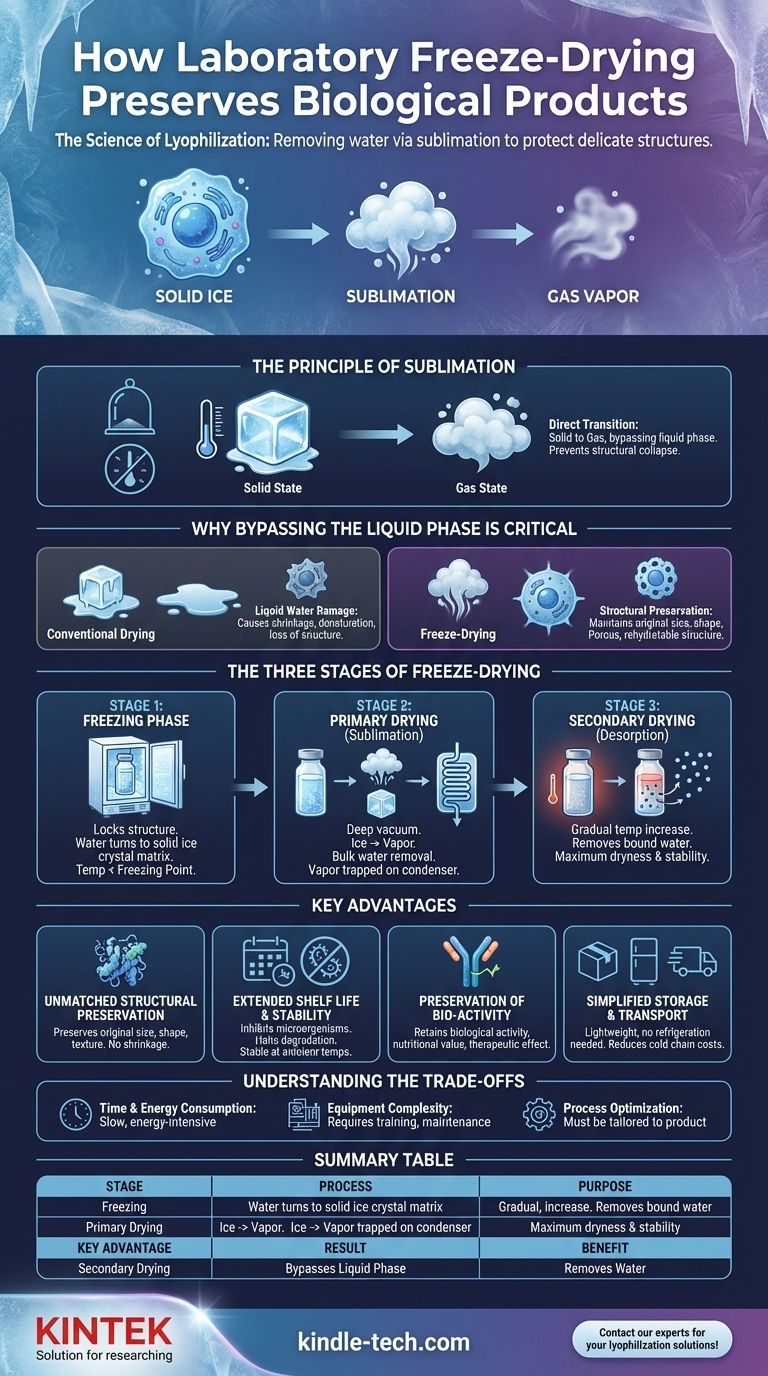
At its core, laboratory freeze-drying preserves biological products by removing water without destroying their delicate structure. The process, formally known as lyophilization, involves freezing the material solid and then using a high-pressure vacuum to transform the ice directly into vapor—a process called sublimation. This bypasses the damaging liquid phase entirely, leaving the product's cellular and chemical architecture intact.
The fundamental advantage of freeze-drying is not just water removal, but the method of removal. By converting solid ice directly to gas, it avoids the physical stress and shrinkage that liquid water causes when leaving a delicate structure, ensuring maximum preservation of the product's original properties.

The Science Behind Lyophilization
Freeze-drying is a multi-stage process governed by a key physical principle. Understanding this principle is crucial to appreciating why it is so effective for sensitive materials like enzymes, vaccines, and cell cultures.
The Principle of Sublimation
Sublimation is the direct transition of a substance from a solid to a gas state, without ever passing through a liquid phase. A common example is dry ice, which turns directly into carbon dioxide gas without melting.
Freeze-dryers create the specific conditions—low temperature and extremely low pressure—where water does the same. This allows water to be extracted from a product as a vapor while the product itself remains frozen solid.
Why Bypassing the Liquid Phase is Critical
When conventional drying methods use heat, liquid water is forced out of the material. This movement can damage and collapse delicate cellular structures, leading to a shrunken, discolored, and often denatured final product.
By sublimating the water, the product's structural backbone remains rigid and in place. The result is a porous, sponge-like structure that can be easily rehydrated later, often returning to its original state with remarkable fidelity.
The Three Stages of Freeze-Drying
Lyophilization is a carefully controlled process broken down into three distinct phases. Each stage serves a specific purpose in gently removing water from the sample.
Stage 1: The Freezing Phase
The first step is to completely freeze the product. The material is brought to a temperature well below its freezing point, ensuring all water is converted into a solid, stable ice crystal matrix.
This initial freezing is a critical step that locks the product's structure in place before the drying process begins.
Stage 2: Primary Drying (Sublimation)
Once frozen, the product is placed under a deep vacuum. This low-pressure environment is the catalyst for sublimation.
As the ice crystals turn to water vapor, this vapor is drawn away from the product and collected on an extremely cold condenser surface inside the freeze-dryer, where it turns back into ice. This effectively traps the removed water, preventing it from re-contaminating the sample.
Stage 3: Secondary Drying (Desorption)
After all the ice crystals have been sublimated, a small amount of "bound" water molecules may remain attached to the product's surface.
In this final stage, the temperature is gradually raised while still under vacuum. This provides just enough energy to break the bonds of these last water molecules, removing them and achieving maximum dryness and long-term stability.
Key Advantages Over Other Preservation Methods
Freeze-drying provides several distinct benefits that make it the superior choice for high-value or delicate biological materials.
Unmatched Structural Preservation
Unlike heat drying which causes shrinkage or simple freezing which can cause damage from large ice crystals, lyophilization preserves the original size, shape, color, and texture of the material.
Extended Shelf Life and Stability
The removal of water inhibits the growth of microorganisms like bacteria and mold. It also halts the enzymatic and chemical reactions that cause degradation, making products stable for years at ambient temperatures.
Preservation of Bio-activity
Because the process avoids high temperatures, sensitive compounds like proteins, enzymes, and antibodies are not denatured. This ensures that their biological activity, nutritional value, and therapeutic effectiveness are retained.
Simplified Storage and Transport
Freeze-dried products are lightweight and do not require refrigeration. This dramatically reduces the cost and complexity of storage and shipping, eliminating the need for an expensive cold chain.
Understanding the Trade-offs
While highly effective, lyophilization is not without its considerations. It is a resource-intensive process that requires careful planning.
Time and Energy Consumption
Freeze-drying is a slow process, often taking many hours or even days to complete. The combination of deep freezing and maintaining a high vacuum consumes a significant amount of energy, making it more costly than other methods.
Equipment Complexity
Laboratory freeze-dryers are sophisticated and expensive instruments. They require proper operator training and regular maintenance to function correctly and achieve consistent, reliable results for sensitive samples.
Process Optimization is Crucial
Freeze-drying is not a one-size-fits-all solution. The parameters for freezing, vacuum level, and temperature must be carefully optimized for each specific product to prevent damage and ensure successful preservation.
Making the Right Choice for Your Goal
The decision to use freeze-drying depends entirely on the nature of your sample and your ultimate objective.
- If your primary focus is long-term stability for vaccines, antibodies, or pharmaceuticals: Freeze-drying is the gold standard for creating a product that is stable at room temperature, easy to transport, and ready for rehydration.
- If your primary focus is preserving the cellular structure of tissues for analysis: Lyophilization is superior for preventing the ice crystal damage and shrinkage common with other dehydration methods.
- If your primary focus is maintaining the nutritional value and bio-activity of enzymes or supplements: This method avoids the high-heat degradation of sensitive compounds that occurs during conventional drying.
By mastering the principles of lyophilization, you can ensure the long-term integrity and viability of your most delicate biological materials.
Summary Table:
| Key Stage | Process | Purpose |
|---|---|---|
| Freezing | Solidifies all water into ice | Locks the product's structure in place |
| Primary Drying | Sublimates ice to vapor under vacuum | Removes the bulk of water without damage |
| Secondary Drying | Removes bound water molecules | Achieves maximum dryness and stability |
| Key Advantage | Result | Benefit |
| Bypasses Liquid Phase | Porous, intact structure | Preserves cellular integrity and bio-activity |
| Removes Water | Lightweight, stable product | Enables ambient storage and transport |
Ready to preserve your most valuable biological samples with precision?
KINTEK specializes in providing reliable laboratory freeze-drying equipment and consumables tailored to your research needs. Our solutions help you achieve maximum sample stability, preserve critical bio-activity, and simplify storage—ensuring your enzymes, vaccines, cell cultures, and pharmaceuticals maintain their integrity for years.
Contact our experts today to find the perfect lyophilization system for your lab!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Liquid Nitrogen Cryogenic Grinder Mill Cryomill Airflow Ultrafine Pulverizer
- Lab Blown Film Extrusion Three Layer Co-Extrusion Film Blowing Machine
People Also Ask
- What is the main difference between freeze drying and vacuum drying? A Guide to Quality vs. Efficiency
- What makes freeze-dried products advantageous for transport? Drastically Reduce Shipping Costs & Simplify Logistics
- What is the technical definition of freeze drying? A Deep Dive into Lyophilization and Sublimation
- What are the hazards of evaporators? Manage Chemical, Thermal, and Pressure Risks
- How should sample volume influence the choice of a lab freeze dryer? A Guide to Capacity, Specs & Cost



















