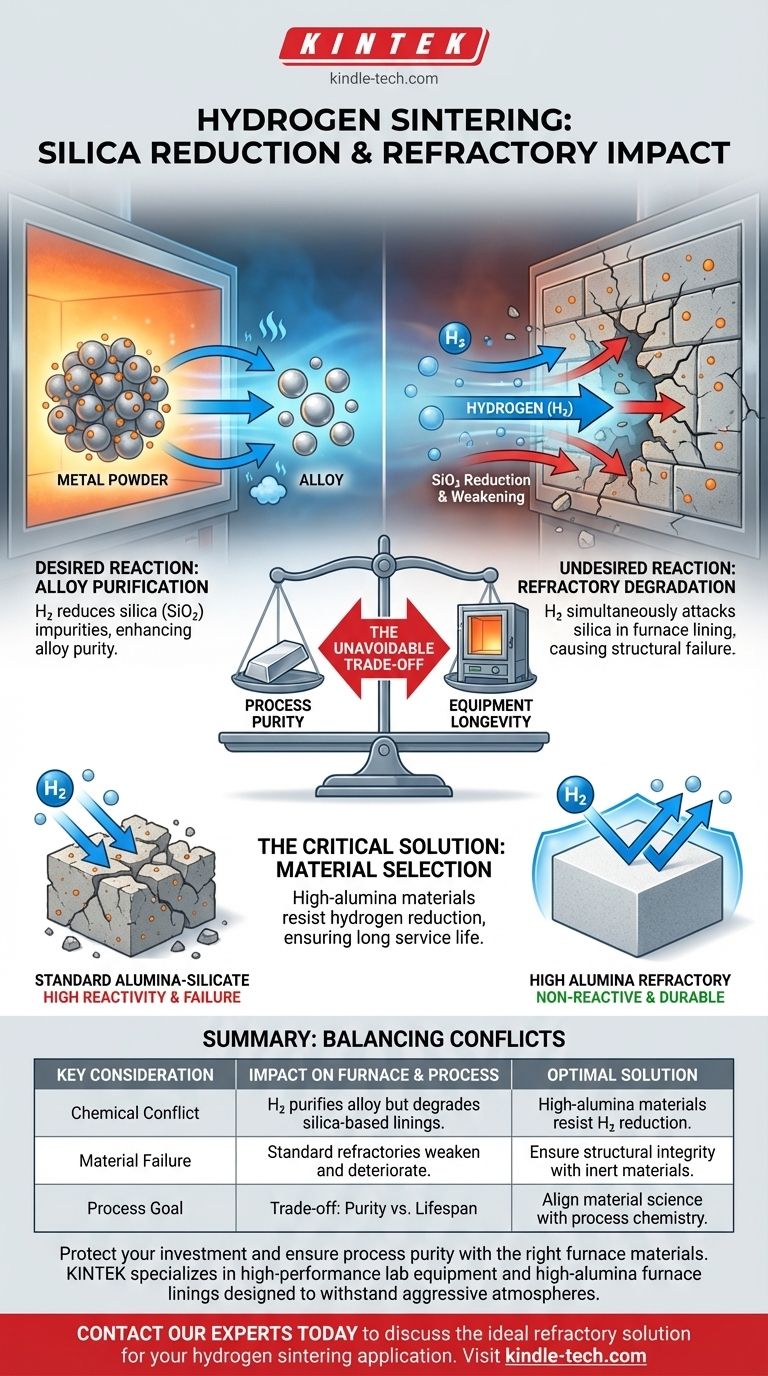
The chemical reduction of silica during hydrogen sintering directly causes the degradation of a furnace's refractory materials. While this chemical reaction is intentionally used to purify the sintered alloy by removing silica impurities, it unintentionally attacks and weakens the furnace lining if it also contains silica-based compounds.
The core issue is a chemical conflict: the same hydrogen atmosphere that purifies the product can simultaneously consume the furnace itself. This makes the selection of a non-reactive refractory material the most critical factor for ensuring furnace longevity and operational stability.

The Underlying Chemical Conflict
To operate a hydrogen sintering furnace effectively, it's essential to understand the two competing reactions happening at high temperatures. One is beneficial for your product, while the other is detrimental to your equipment.
The Desired Reaction: Purifying the Alloy
The primary goal of using a hydrogen atmosphere is often purification. Hydrogen acts as a reducing agent, reacting with oxides like silica (silicon dioxide) present in the metal powder. This reaction removes the oxygen, leaving behind a purer final alloy.
The Undesired Reaction: Degrading the Refractory
The problem arises because the hydrogen atmosphere cannot distinguish between the silica in your product and the silica compounds that may be part of the furnace's refractory lining. The same chemical reduction process that purifies the alloy will attack the structural integrity of the furnace walls, causing them to degrade over time.
Understanding the Trade-offs
This dynamic creates an unavoidable trade-off between process optimization and equipment lifespan. The key is to manage this balance through intelligent material selection.
Process Purity vs. Equipment Longevity
Aggressive sintering conditions, such as higher temperatures or specific hydrogen concentrations designed to maximize silica reduction in the alloy, will inevitably accelerate the degradation of an unsuitable refractory. You gain product purity at the direct cost of your furnace's structural health.
The Critical Role of Material Selection
This conflict makes the choice of refractory material a critical design element, not an afterthought. The material must be fundamentally non-reactive to the hydrogen atmosphere under operational conditions to avoid this degradation cycle entirely.
Selecting the Right Refractory Material
The solution lies in choosing a refractory composition that is inherently stable in a high-temperature hydrogen environment.
Why Common Refractories Can Fail
Many conventional refractory bricks and linings are alumina-silicates, which, as the name implies, contain silica. These materials are highly susceptible to chemical attack and degradation during hydrogen sintering.
The Primary Requirement: Non-Reactivity
The single most important quality for a refractory in this application is its chemical inertness. It must resist reduction by pure or mixed hydrogen atmospheres at the intended sintering temperatures to provide a long service life.
Recommended Materials: High Alumina
For this reason, high alumina or specialized alumina-silicate formulations with very high alumina content are the industry standard. Alumina (aluminum oxide) is significantly more stable and less reactive in hydrogen atmospheres than silica, making it the ideal choice for furnace construction.
Making the Right Choice for Your Furnace
Your operational goals directly inform your material requirements.
- If your primary focus is maximizing product purity: You must invest in high-alumina refractory materials, as standard silica-bearing options will fail quickly under the aggressive conditions required.
- If your primary focus is extending furnace lifespan: The selection of a high-grade, non-reactive refractory is paramount. This ensures the furnace structure remains stable and inert regardless of the sintering process.
Ultimately, aligning your furnace's material science with your process chemistry is the key to achieving both product quality and operational reliability.
Summary Table:
| Key Consideration | Impact on Furnace & Process |
|---|---|
| Chemical Conflict | Hydrogen purifies the alloy but degrades silica-based refractory linings. |
| Material Failure | Standard alumina-silicate refractories weaken and deteriorate over time. |
| Optimal Solution | High-alumina refractory materials resist hydrogen reduction, ensuring longevity. |
Protect your investment and ensure process purity with the right furnace materials.
The chemical conflict in hydrogen sintering demands a refractory solution engineered for stability. KINTEK specializes in high-performance lab equipment and consumables, including furnace linings designed to withstand aggressive atmospheres. Our expertise in high-alumina materials ensures your furnace maintains structural integrity, allowing you to focus on achieving superior product quality without compromising equipment lifespan.
Contact our experts today to discuss the ideal refractory solution for your hydrogen sintering application.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Isostatic Molding Pressing Molds for Lab
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Aluminum Foil Current Collector for Lithium Battery
People Also Ask
- How does an atmosphere sintering furnace contribute to the preparation of LAGP-NCNT composite air electrodes?
- What is the use of hydrogen in furnace? A Key to Oxygen-Free High-Temperature Processing
- What is the difference between modified atmosphere and controlled atmosphere? Mastering Food Preservation Methods
- What is the main hazard associated with the use of inert gases? The Silent Danger of Oxygen Displacement
- What is atmosphere climate control? Master Your Process with a Perfect Environment
- Why is an inert atmosphere pyrolysis furnace required for EVA? Maximize Solar Module Recovery
- What role does a high-temperature hydrogen atmosphere furnace play in the heat treatment of tungsten plates?
- What is a controlled atmosphere furnace? Achieve Purity and Precision in High-Temp Processing



















