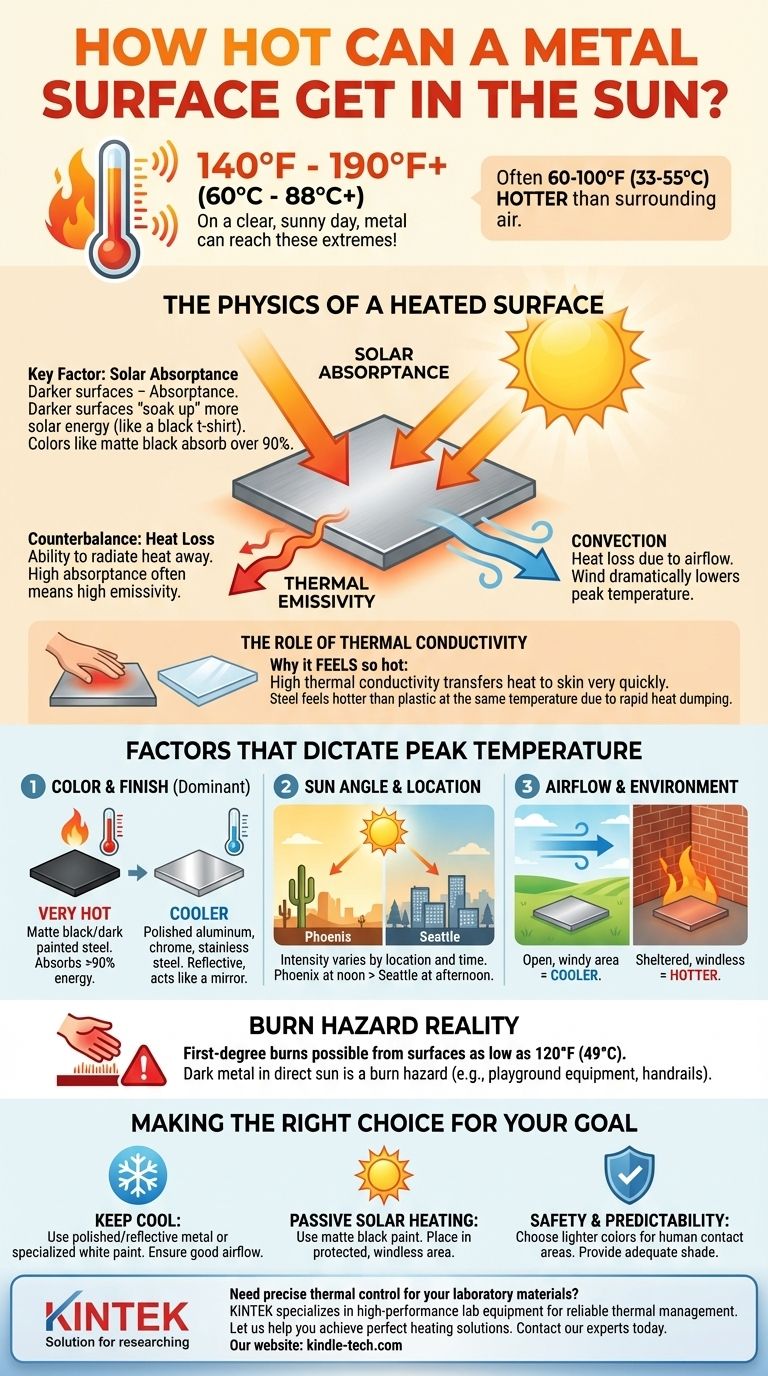
On a clear, hot, sunny day, a metal surface can reach temperatures of 140°F to 190°F (60°C to 88°C) or even higher. The final temperature is not a single number but a dynamic balance, often reaching 60-100°F (33-55°C) hotter than the surrounding air temperature. This extreme heat is determined less by the metal itself and more by its surface finish, color, and exposure to the elements.
The core principle is simple: a metal's temperature in the sun is a battle between energy in and energy out. Its final temperature is the point where the intense energy it absorbs from the sun equals the heat it loses to the surrounding air and through its own radiation.

The Physics of a Heated Surface
To understand why metal gets so hot, you must look beyond the daily weather report. The ambient air temperature is only a secondary factor; the primary driver is direct solar radiation.
The Key Factor: Solar Absorptance
The single most important property is solar absorptance. This is a measure of how much solar energy a surface "soaks up" versus how much it reflects.
Think of it like wearing a black t-shirt versus a white one on a sunny day. The black shirt absorbs most of the light energy and converts it to heat, while the white shirt reflects it. The same is true for metal.
The Counterbalance: Heat Loss
A surface doesn't just absorb heat; it also sheds it. This happens in two main ways.
Thermal emissivity is the ability of a surface to radiate heat away. Coincidentally, surfaces with high absorptance (like matte black paint) also have high emissivity, meaning they are good at both gaining and losing radiant heat.
Convection is heat loss due to airflow. Wind blowing across a metal surface carries heat away, dramatically lowering its peak temperature. A piece of metal will be significantly hotter on a calm day than on a windy one, even if the air temperature is identical.
The Role of Thermal Conductivity
Metal feels exceptionally hot due to its high thermal conductivity. This means it transfers heat to your hand very quickly and efficiently.
A piece of black plastic and a piece of black-painted steel left in the sun may reach the same absolute temperature. However, the steel will feel much hotter and cause a burn more quickly because it can dump its stored heat into your skin far more rapidly.
Factors That Dictate Peak Temperature
Several variables work together to determine the final temperature of a sun-exposed metal object.
Color and Finish
This is the dominant factor. A dark, matte surface will always be hotter than a light, glossy, or polished one.
- Highest Temperatures: Matte black or dark-painted steel. These surfaces can absorb over 90% of solar energy.
- Moderate Temperatures: Unfinished, weathered, or lightly colored metals.
- Lowest Temperatures: Polished aluminum, chrome, or stainless steel. These act like mirrors, reflecting most energy and staying much cooler.
Sun Angle and Location
The intensity of solar radiation changes based on your location and the time of day. A metal roof in Phoenix at high noon in July will get far hotter than a metal bench in Seattle during the late afternoon.
Airflow and Environment
A metal plate in an open, windy field will stay cooler than the same plate located in a sheltered, windless corner next to a brick wall that is also radiating heat.
Common Pitfalls and Misconceptions
Understanding the nuances of thermal dynamics helps avoid common but incorrect assumptions.
Myth: "Metal Just Attracts Heat"
Metal doesn't magically attract heat. It is simply very good at absorbing solar energy (if dark) and extremely effective at conducting that heat to another object (like your hand), which creates the perception of intense heat.
The Emissivity Paradox
It can seem strange that a surface good at absorbing heat (dark and matte) is also good at radiating it away. However, the sun's incoming radiation is so powerful that the absorptance property always wins. The surface gets hot despite also being an efficient radiator.
A shiny surface has low absorptance and low emissivity. It's bad at absorbing heat in the first place, and also bad at radiating away what little heat it does absorb. The net result is that it stays much cooler.
The Burn Hazard Reality
Human skin can suffer first-degree burns from surfaces as low as 120°F (49°C). Since metal can easily surpass this threshold, any dark-colored metal surface in direct sun should be considered a burn hazard, especially for children on playground equipment or for outdoor handrails.
Making the Right Choice for Your Goal
By understanding these principles, you can select materials and finishes to achieve a specific thermal outcome.
- If your primary focus is keeping a surface cool: Use a polished, reflective metal or a specialized white or light-colored paint with high solar reflectance. Ensure the area has good airflow.
- If your primary focus is passive solar heating: Use a metal surface painted matte black to maximize energy absorption and place it in an area protected from wind to minimize convective heat loss.
- If your primary focus is safety and predictability: Choose lighter colors for any metal surfaces intended for human contact, or ensure they are adequately shaded from direct sun.
Ultimately, controlling a metal's temperature is a direct result of managing its surface properties and its environment.
Summary Table:
| Factor | Effect on Temperature | Example |
|---|---|---|
| Color/Finish | Most significant factor | Matte black paint (very hot) vs. polished aluminum (cool) |
| Solar Absorptance | Measures energy absorbed from sun | High absorptance = higher temperature |
| Airflow (Convection) | Cools the surface | Windy day = cooler metal |
| Thermal Emissivity | Ability to radiate heat away | High emissivity helps lose heat |
| Thermal Conductivity | Affects heat transfer feel | Metal feels hotter than plastic at same temperature |
Need precise thermal control for your laboratory materials?
The principles of solar heating—like absorptance, emissivity, and conductivity—are critical in laboratory environments where consistent and accurate temperatures are non-negotiable. KINTEK specializes in high-performance lab equipment and consumables, including ovens, furnaces, and heating elements, designed to provide the reliable thermal management your research demands.
Let us help you achieve perfect heating solutions for your specific application. Contact our experts today to discuss how our products can enhance your lab's efficiency and safety.
Visual Guide
Related Products
- High Purity Gold Platinum Copper Iron Metal Sheets
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Graphite Vacuum Continuous Graphitization Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the methods of synthesis of nanomaterials? Top-Down vs. Bottom-Up Approaches Explained
- What are the different types of frames in compression? A Guide to I, P, and B-Frames
- How is pyrolysis oil used? Unlocking its potential as a fuel and chemical feedstock
- What is bio-oil produced by pyrolysis? A Renewable Fuel Alternative Explained
- What are the chemicals in bio-oil? Unlocking the Complex Chemistry of a Renewable Feedstock
- What is isostatic pressing hot and cold? Forming vs. Finishing for Superior Materials
- How does gold sputter coating work? Achieve Ultra-Thin, Conductive Films for SEM
- What are the limitations of rotary vane pumps? Understanding Oil Dependence and Gas Compatibility
















