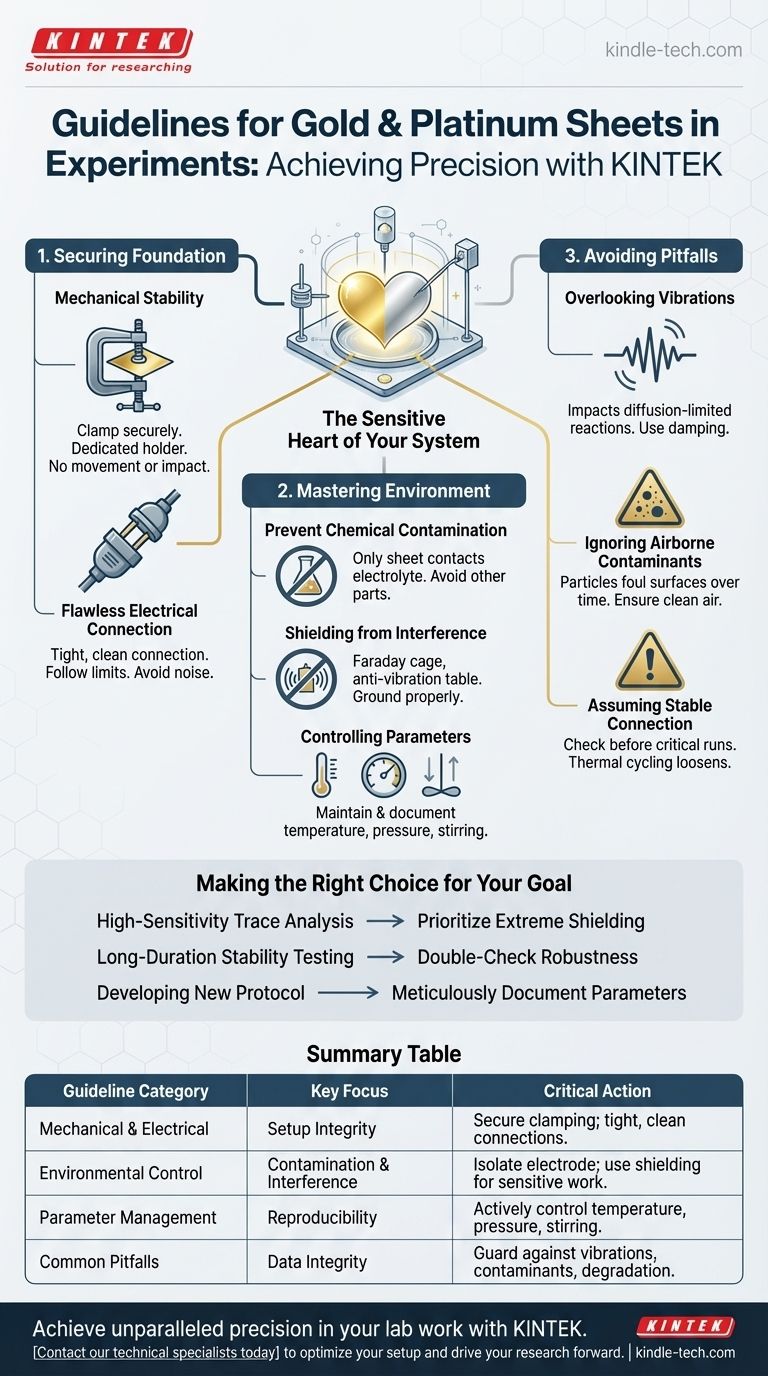
To ensure accurate and repeatable results, the fundamental guidelines for using gold or platinum sheets in an experiment center on three critical areas. You must guarantee the sheet's mechanical stability by clamping it securely, establish a flawless electrical connection to your apparatus, and meticulously control the operating environment to shield it from chemical contamination and physical interference.
The success of your experiment hinges on treating the precious metal sheet not as a simple component, but as the sensitive heart of your measurement system. Rigorous control over its physical, electrical, and chemical environment is the determining factor between ambiguous data and definitive results.

Securing the Physical and Electrical Foundation
Before any measurement begins, the physical and electrical integrity of your setup must be beyond question. Errors at this stage will invalidate all subsequent data.
Proper Mechanical Mounting
Your electrode must be completely immobile. Use a dedicated electrode holder to clamp the sheet securely within your cell or apparatus.
This prevents any movement, falling, or changes in position relative to other electrodes (like reference or counter electrodes), which is critical for consistent electrochemical measurements. Avoid any undue pressure or impact on the sheet during installation.
Ensuring a Flawless Electrical Connection
A poor electrical connection is a primary source of noise and inaccurate readings. Ensure the connection point between the electrode holder and your external apparatus (e.g., a potentiostat) is tight and clean.
Operate within the manufacturer's specified current and voltage limits for your electrode to prevent damage and ensure predictable performance.
Mastering the Experimental Environment
The accuracy of your data is directly tied to how well you can isolate the electrode from external variables. Your goal is to measure only the reaction of interest.
Preventing Chemical Contamination
The electrode surface must only interact with your intended electrolyte. Ensure that no other substances, especially those that could react with gold or platinum, are present.
If you are using a temperature-control system like a water bath, it is imperative that only the gold or platinum portion of the electrode assembly contacts the electrolyte solution. Submerging other parts can lead to contamination and corrosion.
Shielding from External Interference
Precious metal electrodes are highly sensitive probes. Electromagnetic fields from nearby equipment and mechanical vibrations from the building can introduce significant noise and artifacts into your measurements.
Place your setup away from sources of interference. For high-sensitivity work, use a Faraday cage for electromagnetic shielding and an anti-vibration table to dampen physical disturbances. Grounding your apparatus properly is also essential to prevent static buildup.
Controlling Key Experimental Parameters
For results to be reproducible, key conditions must be actively controlled and documented. This includes temperature, pressure, and stirring speed.
While experiments are often best conducted under normal ambient temperature and pressure, your specific protocol dictates these settings. Whatever they are, they must be held constant throughout the measurement period.
Understanding the Common Pitfalls
Even with careful setup, subtle oversights can compromise your experiment. Being aware of these common pitfalls is essential for troubleshooting and ensuring data integrity.
Overlooking "Minor" Vibrations
Even vibrations you can't feel can impact your results. In electrochemical experiments where the reaction rate is limited by diffusion, subtle vibrations can alter the movement of reactants to the electrode surface, changing the measured current and skewing your data.
Ignoring Airborne Contaminants
Contamination doesn't only come from the solution. In environments with poor air quality, airborne particles or volatile organic compounds can settle on and foul your electrolyte or electrode surface over the course of a long experiment.
Assuming a Stable Connection
An electrical connection that was tight at the beginning of an experiment can loosen. Temperature fluctuations can cause materials to expand and contract, potentially degrading the quality of the connection over time. It is good practice to verify connection integrity before each critical run.
Making the Right Choice for Your Goal
Apply these guidelines with a focus on your specific experimental objective.
- If your primary focus is high-sensitivity trace analysis: Prioritize extreme environmental control, including the mandatory use of a Faraday cage and an anti-vibration table.
- If your primary focus is long-duration stability testing: Double-check the robustness of your mechanical mounting and ensure all electrical connections are secured to withstand thermal cycling.
- If your primary focus is developing a new experimental protocol: Meticulously document every parameter—temperature, stirring rate, electrode position, and shielding method—to ensure others can reproduce your work.
Adhering to these principles transforms your experiment from a simple observation into a precise and reliable scientific measurement.
Summary Table:
| Guideline Category | Key Focus | Critical Action |
|---|---|---|
| Mechanical & Electrical | Setup Integrity | Secure clamping with a dedicated holder; ensure tight, clean electrical connections. |
| Environmental Control | Contamination & Interference | Isolate electrode from contaminants; use Faraday cages and anti-vibration tables for sensitive work. |
| Parameter Management | Reproducibility | Actively control and document temperature, pressure, and stirring speed. |
| Common Pitfalls | Data Integrity | Guard against subtle vibrations, airborne contaminants, and connection degradation. |
Achieve unparalleled precision in your lab work with KINTEK.
Your experiments demand the highest level of accuracy and reliability. KINTEK specializes in premium lab equipment and consumables, including electrode holders and shielding solutions, designed to meet the rigorous demands of electrochemical research. We help you eliminate noise, prevent contamination, and ensure reproducible results.
Let us help you optimize your setup. Our experts can provide tailored recommendations to enhance your experimental protocol.
Contact our technical specialists today to discuss your specific needs and discover how KINTEK solutions can drive your research forward.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Gold Electrochemical Sheet Electrode Gold Electrode
- High Purity Gold Platinum Copper Iron Metal Sheets
- Gold Disc Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
People Also Ask
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data