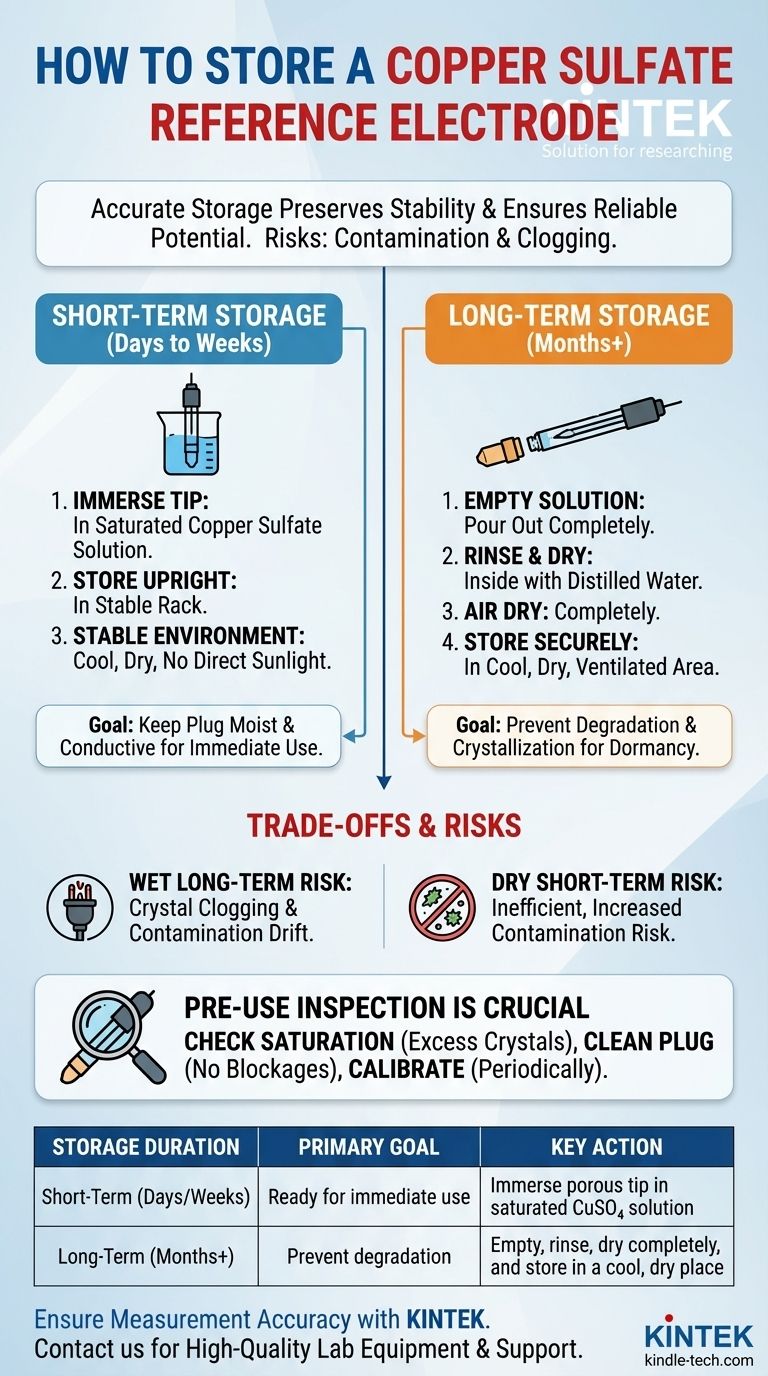
Storing a copper sulfate reference electrode correctly is a matter of time frame. For short-term storage between frequent uses, you should immerse the electrode's porous tip in a saturated copper sulfate solution. For long-term storage, the best practice is to empty the solution, rinse the chamber with distilled water, allow it to dry completely, and then store it in a cool, dry, and protected environment.
The goal of proper storage is not simply to put the electrode away, but to preserve the chemical stability that guarantees an accurate reference potential. The two greatest risks to an electrode's accuracy are contamination of the internal solution and clogging of the porous plug, both of which are prevented by following the correct storage protocol.

The Principle of an Accurate Electrode
To understand why storage matters, you must first understand what makes the electrode work. Its reliability hinges on a stable electrochemical environment.
Why a Stable Potential Matters
A reference electrode works by providing a known, constant potential. The reaction between the internal copper rod and the saturated copper sulfate solution creates this stable benchmark. Any change to the solution's purity or concentration will cause this reference potential to drift, leading to inaccurate measurements.
The Critical Role of the Porous Plug
The ceramic or wooden plug at the tip of the electrode is the gatekeeper. It allows electrical contact with the soil or electrolyte you are measuring but prevents the internal solution from leaking out or becoming contaminated by outside elements. A clogged or compromised plug will obstruct this electrical path and render your readings invalid.
Storage Protocols for Optimal Performance
Your storage method should be dictated by how frequently you plan to use the electrode.
Short-Term Storage (Days to Weeks)
For an electrode in regular use, the goal is to keep it ready for immediate deployment.
- Immerse the Tip: Place the electrode in a small container with just enough saturated copper sulfate solution to keep the porous plug submerged.
- Store Upright: Keep the electrode upright in a stable rack to prevent it from tipping over.
- Find a Stable Environment: Store it in a cool, dry place away from direct sunlight and significant temperature fluctuations.
This method keeps the plug moist and conductive while maintaining the chemical equilibrium inside the electrode.
Long-Term Storage (Months or Longer)
If you are storing the electrode for the off-season or an extended period, you must prepare it for dormancy to prevent degradation.
- Empty the Solution: Carefully pour out all of the copper sulfate solution.
- Rinse Thoroughly: Rinse the inside of the electrode chamber with distilled water to remove any residual sulfate crystals.
- Air Dry Completely: Allow the electrode and its components to air dry fully. Any trapped moisture can lead to corrosion or unwanted chemical reactions.
- Store Securely: Place the dry electrode in a cool, dry, and well-ventilated area. Using its original box is ideal to protect it from physical damage and dust.
Understanding the Trade-offs and Risks
Choosing the wrong storage method introduces predictable failures. Understanding these trade-offs is key to maintaining your equipment.
Risk of Storing "Wet" Long-Term
Leaving the solution in the electrode for many months is a common mistake. Water will slowly evaporate, causing copper sulfate crystals to form inside and clog the porous plug. The solution is also more susceptible to gradual contamination over time, causing potential drift.
Risk of Storing "Dry" Short-Term
While safer for long periods, the "dry" method is impractical for daily or weekly use. The constant cycle of emptying, rinsing, drying, and refilling is inefficient and increases the chances of introducing contaminants during handling.
Environmental Hazards
Always store the electrode away from high temperatures, high humidity, and corrosive gases. These conditions can degrade the electrode's plastic or glass body, corrode its connections, and compromise the integrity of your equipment.
Pre-Use Inspection is Crucial
After storage, always inspect your electrode. The transparent body allows you to confirm the solution is saturated, which is indicated by the presence of excess, undissolved copper sulfate crystals at the bottom. Also, check that the porous plug is clean and free of blockages before taking any critical measurements. If you doubt your readings, a quick calibration check against a trusted second electrode is a wise step.
Making the Right Choice for Your Goal
Your storage method should directly support your operational needs.
- If your primary focus is frequent field use (daily or weekly): Use the short-term "wet" storage method by keeping the tip immersed in a saturated copper sulfate solution to ensure it is always ready.
- If your primary focus is preserving the electrode for next season: Use the long-term "dry" storage method by emptying, rinsing, and drying the electrode completely to prevent crystallization and contamination.
- If your primary focus is measurement accuracy: Always visually inspect the solution and plug before use, and periodically calibrate the electrode against a known standard to confirm its potential has not drifted.
Proper storage is the most critical maintenance you can perform to ensure your measurements are reliable and your electrode has a long, accurate service life.
Summary Table:
| Storage Duration | Primary Goal | Key Action |
|---|---|---|
| Short-Term (Days/Weeks) | Ready for immediate use | Immerse porous tip in saturated CuSO₄ solution |
| Long-Term (Months+) | Prevent degradation | Empty, rinse, dry completely, and store in a cool, dry place |
Ensure your laboratory's measurement accuracy with reliable equipment from KINTEK.
Properly storing your reference electrodes is just one part of maintaining precise results. KINTEK specializes in high-quality lab equipment and consumables, providing the tools and support your laboratory needs for reliable, accurate performance.
Let our experts help you select the right equipment for your specific application. Contact KINTEK today to discuss your laboratory requirements and discover how we can support your success.
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- How should a copper sulfate reference electrode be maintained? Ensure Accurate Electrochemical Measurements
- What is the operating principle of a copper sulfate reference electrode? Reliable Potential Measurement Explained
- What are the post-treatment procedures after using a copper sulfate reference electrode? Essential Steps for Accuracy & Longevity
- What precautions should be taken when handling and using a copper sulfate reference electrode? Ensure Accurate Electrochemical Measurements
- What are the pre-treatment steps before using a portable copper sulfate reference electrode? Ensure Accurate Corrosion Potential Measurements



















