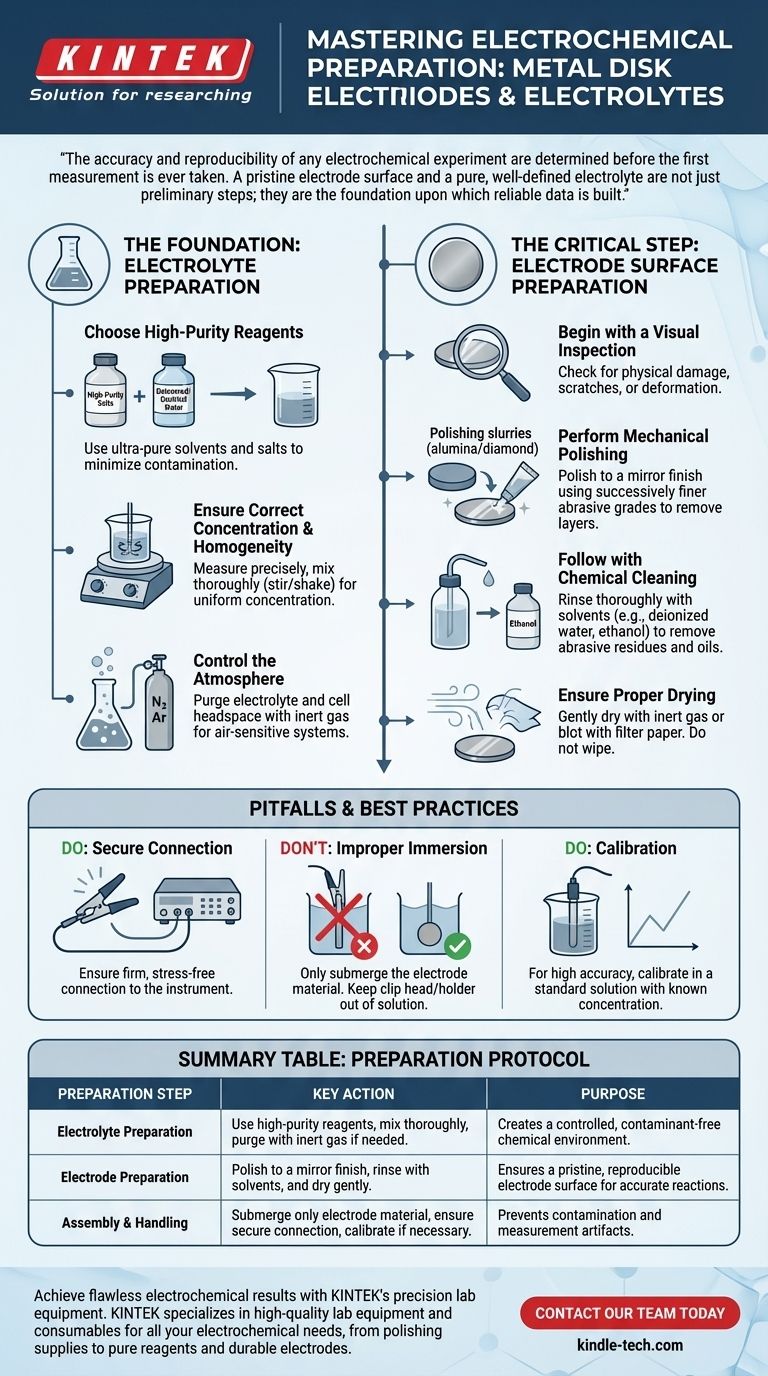
To properly prepare a metal disk electrode and its electrolyte, you must first meticulously clean the electrode surface and then separately prepare a pure, homogeneous electrolyte solution. The electrode is typically polished, rinsed with solvents like deionized water or ethanol to remove all contaminants, and dried, while the electrolyte is formulated with high-purity reagents and thoroughly mixed to ensure uniform concentration.
The accuracy and reproducibility of any electrochemical experiment are determined before the first measurement is ever taken. A pristine electrode surface and a pure, well-defined electrolyte are not just preliminary steps; they are the foundation upon which reliable data is built.

The Foundation: Preparing the Electrolyte
An impure or improperly mixed electrolyte is a primary source of experimental error. Each step in its preparation is designed to create a controlled and predictable chemical environment.
Choose High-Purity Reagents
The first step is selecting the appropriate materials. Any impurities in the chemical reagents or the solvent can introduce unintended electrochemical reactions.
Always use high-purity salts and solvents, such as deionized or distilled water, to minimize contamination that could interfere with your measurements.
Ensure Correct Concentration and Homogeneity
The concentration of your electrolyte directly impacts conductivity and reaction kinetics. Inaccurate concentrations lead to invalid results.
After carefully measuring and mixing the components, the solution must be thoroughly stirred or shaken. This ensures it is completely homogeneous and the concentration is uniform throughout the cell.
Control the Atmosphere
Many electrochemical reactions are sensitive to atmospheric gases, particularly oxygen, which is electrochemically active.
If your experiment is air-sensitive, you must purge the electrolyte and headspace within the electrochemical cell with an inert gas, such as nitrogen or argon, before introducing the electrodes.
The Critical Step: Preparing the Electrode Surface
The electrode surface is where the chemical reaction of interest occurs. Any contamination, oxide layer, or surface irregularity can block or alter this reaction, invalidating your data.
Begin with a Visual Inspection
Before any treatment, examine the electrode. Look for obvious physical damage, deep scratches, or deformation that could compromise its performance or sealing within the cell.
Perform Mechanical Polishing
For most solid metal disk electrodes, a fresh, reproducible surface is created by mechanical polishing. This process removes any existing oxide layers and surface contaminants from previous experiments.
This is typically done using polishing pads with successively finer grades of alumina or diamond slurry to achieve a mirror-like finish.
Follow with Chemical Cleaning
After polishing, the surface must be cleaned of any residual polishing abrasive and oils.
Thoroughly rinse the electrode surface with a solvent like deionized water, followed by ethanol or another suitable solvent, to remove all particulate matter and organic grease.
Ensure Proper Drying
Finally, gently dry the electrode surface. You can use a stream of inert gas or carefully blot it with a lint-free tissue, such as filter paper. Avoid wiping, which can re-contaminate the clean surface.
Understanding the Pitfalls and Best Practices
Meticulous preparation can be undone by simple mistakes during assembly. Proper handling ensures the integrity of your carefully prepared system.
The Danger of Improper Immersion
When placing the electrode into the cell, ensure that only the intended electrode material is submerged in the electrolyte.
The clip head or holder, which connects the electrode to the instrument, should never touch the solution. These parts often have solder points sealed with adhesive that can be damaged by the electrolyte, leading to contamination and electrode failure.
The Need for a Secure Connection
Once in the cell, the electrode must be securely connected to the potentiostat. A loose or intermittent connection will introduce significant noise and artifacts into your measurements.
Ensure the connection is firm but do not apply mechanical stress, such as bending or twisting, to the electrode body.
The Role of Calibration
For experiments requiring high accuracy, such as analytical measurements, calibration may be necessary.
This involves running your prepared electrode in a standard solution with a known concentration to verify its response according to the instrument's instructions.
Making the Right Choice for Your Experiment
Your preparation protocol should match the demands of your experiment.
- If your primary focus is qualitative analysis or a quick screening: A thorough solvent cleaning to remove surface grease and oxides may be sufficient.
- If your primary focus is quantitative measurement or kinetic studies: A full protocol of mechanical polishing, solvent cleaning, and drying is essential for reproducibility.
- If your primary focus is studying an air-sensitive system: You must incorporate electrolyte and cell purging with an inert gas into your standard preparation routine.
Ultimately, investing time in a rigorous and consistent preparation protocol is the most effective way to ensure the quality and integrity of your electrochemical data.
Summary Table:
| Preparation Step | Key Action | Purpose |
|---|---|---|
| Electrolyte Preparation | Use high-purity reagents, mix thoroughly, and purge with inert gas if needed. | Creates a controlled, contaminant-free chemical environment. |
| Electrode Preparation | Polish to a mirror finish, rinse with solvents (e.g., deionized water, ethanol), and dry gently. | Ensures a pristine, reproducible electrode surface for accurate reactions. |
| Assembly & Handling | Submerge only the electrode material, ensure a secure connection, and calibrate if necessary. | Prevents contamination and measurement artifacts. |
Achieve flawless electrochemical results with KINTEK's precision lab equipment.
Proper preparation is the foundation of reliable data, and having the right tools is critical. KINTEK specializes in high-quality lab equipment and consumables for all your electrochemical needs, from polishing supplies to pure reagents and durable electrodes.
Let our expertise support your research. Contact our team today to discuss your specific application and ensure your lab is equipped for success.
Visual Guide
Related Products
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Hexagonal Boron Nitride HBN Ceramic Ring
- Laboratory Grinding Mill Mortar Grinder for Sample Preparation
People Also Ask
- What is grinder in chemistry? A Guide to Precision Sample Preparation
- What is the difference between mixer and disperser? Choose the Right Tool for Your Process
- How does a constant temperature rotary shaker contribute to evaluating iron nanoparticles? Optimize Dye Degradation
- What is the function of high-shear dispersion equipment in corona-resistant nanocomposites? Elevate Your Insulation
- What is a laboratory mixer? A Guide to Achieving Perfect Sample Homogeneity



















