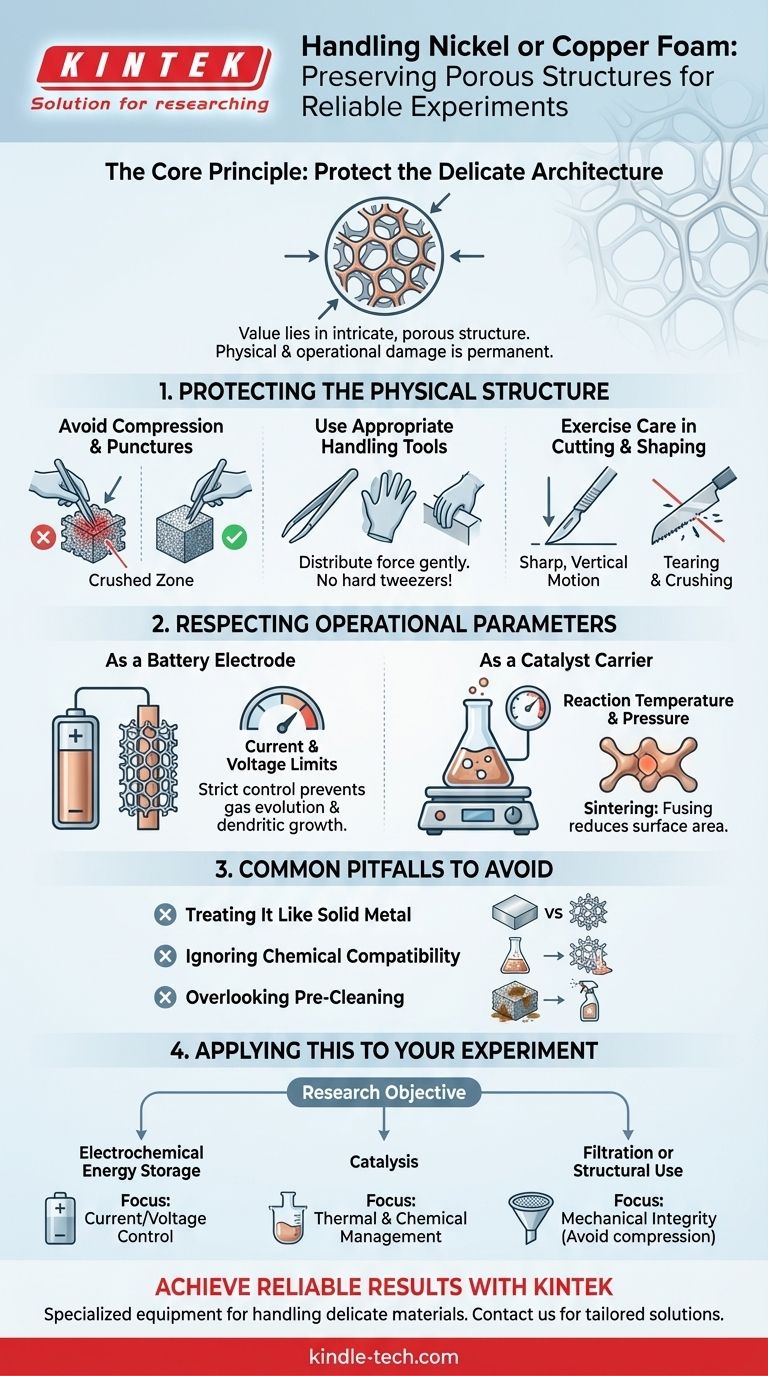
In essence, you must handle nickel or copper foam with extreme care, protecting it from both physical damage and operational stress. The goal is to preserve its delicate, three-dimensional porous structure, which is the source of its unique properties. Mishandling, whether by scratching it or exceeding its intended thermal or electrical limits, can irreversibly compromise this structure and invalidate your experimental results.
The core principle is simple: the value of metal foam lies entirely in its intricate, porous architecture. Physical compression crushes this structure, while exceeding operational parameters chemically or thermally degrades it. Both forms of damage permanently destroy the material's high surface area and performance capabilities.

Protecting the Physical Structure
The most immediate risk is physical damage. Unlike a solid piece of metal, metal foam has a very low density and is defined by its voids. Protecting this structure is paramount.
Avoid Compression and Punctures
Any act of compressing, scratching, or puncturing the foam with a sharp object is a critical failure. This action permanently crushes the interconnected pores in the affected area.
This damage is not cosmetic. It eliminates the high surface area and permeability that you are relying on for your work, effectively creating a "dead zone" in the material.
Use Appropriate Handling Tools
Never use hard or sharp metal tweezers to handle the foam. The pressure from the tips will easily crush the ligaments of the porous network.
Instead, use soft-tipped tweezers, nitrile or latex gloves, or gently handle the material by its edges. The goal is to distribute any handling force as widely and gently as possible.
Exercise Care in Cutting and Shaping
If you must cut the foam to size, use a very sharp, fresh razor blade or scalpel. Press down firmly and vertically in a single motion rather than using a sawing action.
A sawing motion or a dull blade will tear and crush the pores along the cut line, compromising the edges of your sample.
Respecting the Operational Parameters
Physical handling is only half the challenge. The foam's structure is also vulnerable to its experimental environment. You must operate strictly within the material's specified limits.
As a Battery Electrode
When using nickel or copper foam as an electrode scaffold, the charge/discharge current and voltage window are critical parameters.
Exceeding these limits can lead to unwanted side reactions, gas evolution that mechanically stresses the structure, or dendritic growth that can short-circuit a cell and physically damage the foam.
As a Catalyst Carrier
For catalysis applications, reaction temperature and pressure must be carefully controlled. Excessively high temperatures can cause the metal to sinter.
Sintering is a process where the fine metal ligaments begin to fuse, reducing the overall surface area and, therefore, the catalytic activity. Similarly, high pressures or aggressive reactants can degrade the foam's structure over time.
Common Pitfalls to Avoid
Understanding what not to do is as important as knowing what to do. Many promising experiments fail due to simple, avoidable mistakes in material handling.
Treating It Like Solid Metal
The most common mistake is to perceive the foam as a robust metal sponge. It is not. It is a high-tech material whose properties are directly tied to its delicate and precise architecture.
Ignoring Chemical Compatibility
While nickel and copper are relatively stable, they are not inert. Ensure your experimental medium, including solvents and electrolytes, is not overly corrosive to the base metal unless etching is a specific, controlled part of your procedure.
Overlooking Pre-Cleaning
As-received metal foam often has residual oils from manufacturing or a thin native oxide layer. Failing to clean the foam with appropriate solvents (like acetone or isopropanol) or perform a pre-treatment can lead to poor performance and non-repeatable results.
Applying This to Your Experiment
Your handling strategy should be directly informed by your research objective.
- If your primary focus is electrochemical energy storage (batteries/capacitors): Your priority is precise control over current and voltage to prevent physical degradation from plating or gas evolution.
- If your primary focus is catalysis: Your priority is strict thermal and chemical management to prevent sintering or corrosion, which would destroy the active surface area.
- If your primary focus is filtration or structural use: Your priority is mechanical integrity, avoiding any compression that would alter permeability and flow characteristics.
Ultimately, disciplined handling ensures the material's unique structure works for you, not against you, yielding reliable and conclusive data.
Summary Table:
| Handling Aspect | Key Consideration | Common Mistake to Avoid |
|---|---|---|
| Physical Handling | Use soft-tipped tools, handle by edges | Compressing with metal tweezers |
| Cutting/Shaping | Use a sharp blade, single vertical cut | Sawing motion with a dull blade |
| Electrochemical Use | Strictly control current/voltage limits | Exceeding limits causing gas evolution |
| Catalytic Use | Manage temperature to prevent sintering | Overheating that fuses metal ligaments |
Achieve Reliable and Repeatable Results with KINTEK
Handling delicate materials like nickel or copper foam requires precision and the right equipment. KINTEK specializes in providing high-quality lab equipment and consumables tailored for advanced material research. Whether you are working on battery electrodes, catalysis, or filtration, our products are designed to help you maintain the integrity of your samples and ensure experimental success.
Let our expertise support your innovation. Contact our team today to discuss how KINTEK can meet your specific laboratory needs and help you protect your valuable research materials.
Visual Guide
Related Products
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Electrode Polishing Material for Electrochemical Experiments
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
- Custom PTFE Teflon Parts Manufacturer for Hollow Etching Flower Basket ITO FTO Developing Glue Removal
- Custom PTFE Teflon Parts Manufacturer for PTFE Containers
People Also Ask
- What are 3 products that carbon nanotubes can be used in? Enhancing Batteries, Tires, and Composites
- Why are high surface area materials preferred for BES anodes? Maximize Microbial Power and Efficiency
- What are the material properties of carbon paper? Unlocking High Conductivity & Porosity for Your Lab
- What can carbon nanotubes be used for? Unlock Superior Performance in Batteries & Materials
- How should carbon cloth used for high-temperature electrolysis be handled after operation? Prevent Irreversible Oxidative Damage



















