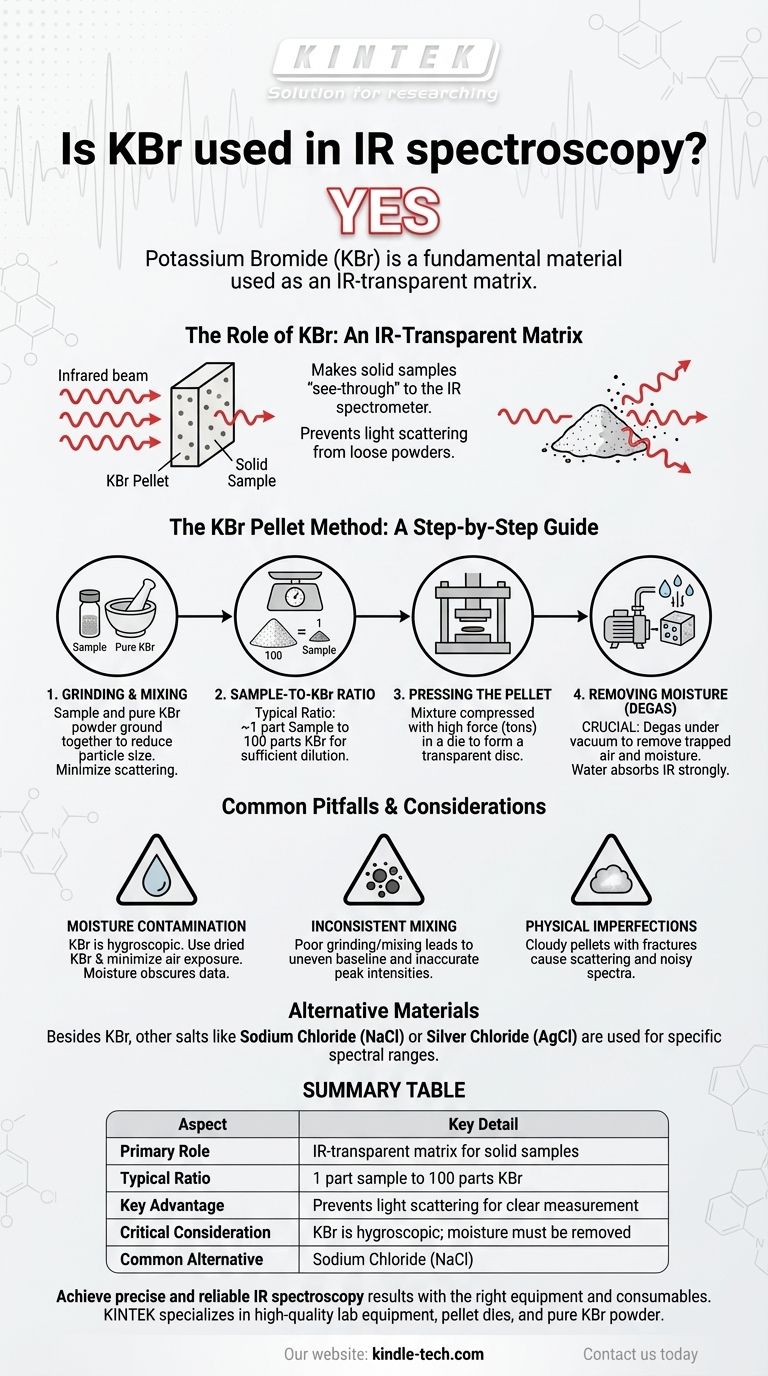
Yes, Potassium Bromide (KBr) is a fundamental material used in IR spectroscopy. It is one of the most common and effective methods for preparing solid samples for analysis. The technique involves mixing a small amount of the solid sample with pure KBr powder and compressing it into a thin, transparent disc or "pellet."
The core purpose of KBr is to act as an IR-transparent matrix. It holds the solid sample in the path of the infrared beam without producing its own interfering signals, effectively making the solid "see-through" to the spectrometer.

The Role of KBr in Analyzing Solids
Infrared spectroscopy works by passing IR radiation through a substance. While analyzing liquids and gases is straightforward, analyzing a solid powder directly is often impossible because the crystals scatter the light, preventing a clean measurement.
Why KBr is the Solution
Potassium Bromide (KBr) and other alkali halides possess a unique physical property: under high pressure, they become plastic and flow to form a solid, glass-like sheet. This sheet is transparent to infrared radiation across a wide spectral range.
By mixing a powdered sample into the KBr before pressing, the sample becomes evenly dispersed within this IR-transparent matrix. This allows the spectrometer's beam to pass through the sample for analysis without the scattering issues associated with a loose powder.
The Sample-to-KBr Ratio
The sample must be sufficiently diluted to allow enough light to pass through for detection. A typical preparation uses a ratio of approximately 1 part sample to 100 parts KBr.
The KBr Pellet Method Explained
Creating a high-quality KBr pellet is a precise process. Each step is designed to produce a clear, homogeneous disc that will yield a clean and accurate spectrum.
Step 1: Grinding and Mixing
First, the sample and the KBr powder are ground together, typically with a mortar and pestle. This step is critical to reduce the particle size of the sample, which minimizes light scattering and ensures it is uniformly distributed within the KBr.
Step 2: Pressing the Pellet
The fine powder mixture is then placed into a pellet die. The die is placed in a hydraulic press and compressed with several tons of force. This immense pressure causes the KBr to fuse into a solid, transparent disc containing the sample.
Step 3: Removing Moisture
Degassing the die under a vacuum before and during pressing is a crucial step. This removes trapped air and, most importantly, moisture. Water has very strong absorption bands in the infrared spectrum and will obscure important regions of the sample's data if not removed.
Common Pitfalls and Considerations
While effective, the KBr pellet method requires care. Several factors can lead to a poor-quality spectrum, and understanding them is key to successful analysis.
Moisture Contamination
KBr is hygroscopic, meaning it readily absorbs moisture from the air. This is the most common source of error. Using dried KBr and minimizing exposure to the atmosphere is essential for a clean background spectrum.
Inconsistent Sample Distribution
If the sample is not ground finely or mixed thoroughly, it can clump together within the pellet. This leads to an uneven baseline and inaccurate peak intensities in the final spectrum.
Physical Pellet Imperfections
A poorly prepared pellet may appear cloudy or contain fractures. This cloudiness is caused by large particles that scatter the IR beam, reducing the signal quality and making the spectrum noisy.
Alternative Materials
While KBr is the most common choice, other salts like Sodium Chloride (NaCl) or Silver Chloride (AgCl) can also be used. The choice of material depends on the specific spectral range being investigated and the chemical reactivity of the sample.
Making the Right Choice for Your Goal
Using KBr is a standard technique, but its success depends on careful preparation aligned with your analytical needs.
- If your primary focus is obtaining a high-quality, routine spectrum of a stable solid: The KBr pellet method is the gold standard and most cost-effective approach.
- If you are troubleshooting a noisy or inaccurate spectrum: First, suspect moisture contamination or insufficient grinding of your sample.
- If your sample is sensitive to high pressure or reacts with KBr: You will need to investigate alternative sample preparation methods beyond the scope of this technique.
Ultimately, mastering the KBr pellet technique is a crucial skill for accurate and repeatable infrared analysis of solid materials.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Primary Role | IR-transparent matrix for solid samples |
| Typical Ratio | 1 part sample to 100 parts KBr |
| Key Advantage | Prevents light scattering for clear measurement |
| Critical Consideration | KBr is hygroscopic; moisture must be removed |
| Common Alternative | Sodium Chloride (NaCl) for different spectral ranges |
Achieve precise and reliable IR spectroscopy results with the right equipment and consumables. KINTEK specializes in high-quality lab equipment and consumables, including reliable pellet dies and pure KBr powder, to support your laboratory's needs for accurate solid sample analysis. Contact us today via our [#ContactForm] to discuss how our solutions can enhance your spectroscopic workflow and ensure repeatable success.
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- kbr pellet press 2t
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- Which is better EDX or XRF? Choose the Right Elemental Analysis Tool for Your Needs
- Can a hydraulic press press anything? Understanding the Real Limits of Its Power
- What is the compression ratio of a pellet mill die? The Key to Durable, High-Quality Pellets
- What is the primary function of a laboratory hydraulic press in the preparation of graphite electrodes?
- Can a diamond break under a hydraulic press? Uncover the Truth About Diamond's Brittleness
- What role does a laboratory hydraulic press play in the preparation of solid electrolyte pellets? Ensure Data Accuracy
- What is the mechanism by which a laboratory hydraulic press facilitates the sintering of TiB2-SiC? Optimize Density
- What is KBr method in IR spectroscopy? Master Solid Sample Analysis for Clear IR Spectra



















