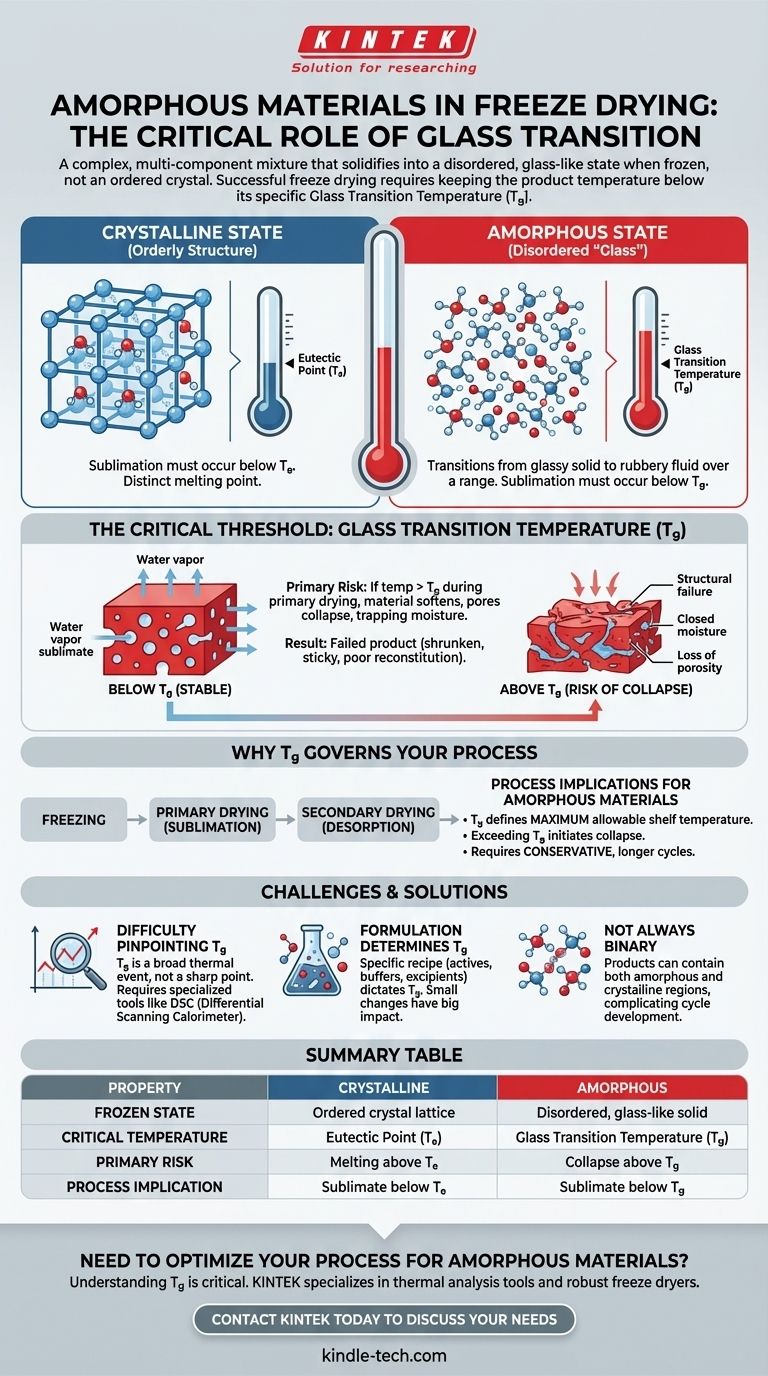
In the context of freeze drying, an amorphous material is a complex, multi-component mixture that does not form an orderly crystal structure when frozen. Instead, it solidifies into a disordered, glass-like state. This physical state is fundamentally different from crystalline materials, and successful freeze drying requires keeping the product temperature below its specific "glass transition temperature" to prevent structural failure.
The core distinction between freezing a simple substance like pure water and a complex formulation is the state it forms. While crystalline materials have a distinct melting point, amorphous "glassy" materials have a glass transition temperature (Tg), and respecting this threshold is the single most critical factor for a successful freeze-drying cycle.

The Fundamental Difference: Glass vs. Crystal
The success of any freeze-drying (lyophilization) process depends on understanding the physical nature of your frozen product. The primary distinction is whether it forms a crystalline lattice or an amorphous glass.
The Crystalline State: An Orderly Structure
Crystalline materials, when frozen, arrange themselves into a highly ordered, solid structure.
These materials have a eutectic point (Te). This is the single, lowest temperature at which a mixture of components will melt. For freeze drying to work, the sublimation process must occur below this temperature.
The Amorphous State: A Disordered "Glass"
Amorphous materials lack the ability to form an ordered crystal lattice, often due to the complexity of the molecules in the mixture.
Instead of crystallizing, the solution becomes increasingly viscous as it cools, eventually locking into a solid but disordered state. This is known as a vitrified or "glassy" state.
The Glass Transition Temperature (Tg): The Critical Threshold
Amorphous materials do not have a eutectic point. Instead, they have a glass transition temperature (Tg).
This is not a sharp melting point but a temperature range over which the material transitions from a hard, glassy solid to a soft, rubbery, and highly viscous fluid. For sublimation to proceed correctly, the product's temperature must remain below its Tg.
Why This Distinction Governs Your Freeze-Drying Process
Knowing whether your material is amorphous or crystalline dictates your entire process strategy, especially the parameters for primary drying, where the bulk of the water is removed via sublimation.
The Primary Risk: Product "Collapse"
If an amorphous product's temperature exceeds its glass transition temperature (Tg) during primary drying, it begins to soften.
This softening causes the microscopic pore structure of the solid matrix to break down or "collapse." The pathways needed for water vapor to escape are closed off, trapping the remaining moisture.
A collapsed product is a failed product. It often appears shrunken, sticky, or gummy and will not properly reconstitute.
Defining Your Process Parameters
The three stages of freeze drying—freezing, primary drying (sublimation), and secondary drying (desorption)—are all influenced by the material's state.
For an amorphous product, the Tg defines the maximum allowable shelf temperature during primary drying. Exceeding it, even briefly, can initiate collapse. This is why cycles for amorphous products are often more conservative and longer than for high-eutectic crystalline materials.
Understanding the Trade-offs and Challenges
While the concept is straightforward, working with amorphous materials presents unique challenges that require careful management.
The Difficulty of Pinpointing Tg
Unlike a sharp eutectic point, the glass transition is a broader thermal event. Accurately identifying the precise Tg of your formulation is critical and often requires specialized thermal analysis tools like a Differential Scanning Calorimeter (DSC).
Formulation Determines Everything
The specific components of your mixture—the active ingredient, sugars, salts, and buffering agents—all contribute to the final Tg of the formulation. Small changes in the recipe can significantly raise or lower this critical temperature.
Not Always a Clear Binary
Some products are not purely amorphous or crystalline. They can contain regions of both, which complicates the development of a freeze-drying cycle, as you must respect the constraints of both states.
Making the Right Choice for Your Process
Your approach to developing a freeze-drying cycle must be guided by the physical properties of your frozen material.
- If your primary focus is on a known crystalline material: Your main objective is to identify its eutectic point (Te) and ensure the product temperature remains below this value during sublimation.
- If your primary focus is on an amorphous material: You must determine the glass transition temperature (Tg) and meticulously control the process to keep the product below this threshold to prevent collapse.
- If you are unsure of your material's state: It is critical to perform thermal analysis before developing a cycle, as running a process with incorrect temperature targets is the most common cause of product failure.
Understanding whether your material forms a glass or a crystal is the foundational step to designing a stable, efficient, and successful lyophilization process.
Summary Table:
| Property | Crystalline Material | Amorphous Material |
|---|---|---|
| Frozen State | Ordered crystal lattice | Disordered, glass-like solid |
| Critical Temperature | Eutectic Point (Te) | Glass Transition Temperature (Tg) |
| Primary Risk | Melting above Te | Collapse above Tg |
| Process Implication | Sublimate below Te | Sublimate below Tg |
Need to optimize your freeze-drying process for amorphous materials?
Understanding the glass transition temperature (Tg) of your formulation is critical to preventing product collapse and ensuring a stable, high-quality final product. KINTEK specializes in providing the laboratory equipment and consumables you need for successful lyophilization, from thermal analysis tools to robust freeze dryers.
Let our experts help you design an efficient and reliable process tailored to your specific amorphous materials. Contact KINTEK today to discuss your laboratory needs and achieve superior freeze-drying results.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Liquid Nitrogen Cryogenic Grinder Mill Cryomill Airflow Ultrafine Pulverizer
- Touchscreen Automatic Vacuum Heat Press
People Also Ask
- What are some do's and don'ts when using a Laboratory Freeze Dryer? Master the Core Principles for Success
- What are the three main stages of the freeze-drying process? Master Sublimation and Desorption
- What are the main steps involved in the freeze-drying process? A Guide to the 3 Key Stages
- What factors should be considered when purchasing a freeze dryer? Match Your Needs for Optimal Performance & Value
- What makes freeze-dried products advantageous for transport? Drastically Reduce Shipping Costs & Simplify Logistics



















