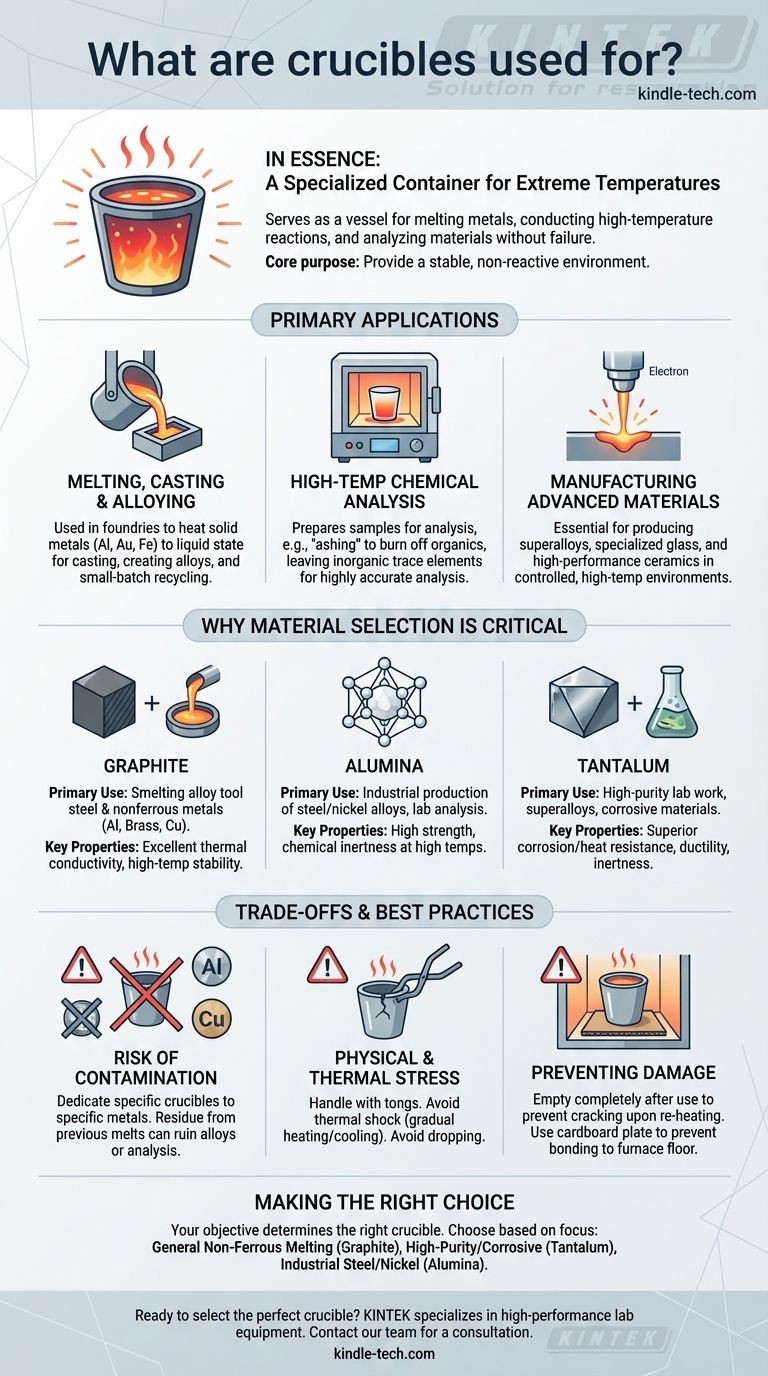
In essence, a crucible is a specialized container engineered to withstand extreme temperatures. It serves as a vessel for melting metals, conducting high-temperature chemical reactions, and analyzing materials without the container itself melting, breaking, or reacting with its contents. This function is fundamental across metallurgy, chemistry, and materials science.
The core purpose of a crucible is to provide a stable, non-reactive environment for substances at temperatures that would destroy ordinary containers. The specific material of the crucible—from graphite to tantalum—is chosen to match the precise thermal and chemical demands of the task.

The Primary Applications of Crucibles
A crucible's design is deceptively simple, but its role is critical in several high-stakes processes. Its primary function is always to contain and isolate a substance during intense heating.
Melting, Casting, and Alloying Metals
In metallurgy and foundries, crucibles are indispensable. They are used to heat solid metals like aluminum, gold, or iron past their melting point into a liquid state.
This molten metal can then be poured into molds for casting, used to create alloys by mixing with other molten metals, or used in small-batch scrap metal recycling.
High-Temperature Chemical Analysis
In laboratory settings, particularly in analytical chemistry, crucibles are used to prepare samples for analysis. A common technique is "ashing," where a sample is heated to burn off all organic matter, leaving only inorganic trace elements behind.
Because the crucible is chemically inert, it ensures that the only material left is from the original sample, allowing for highly accurate trace and ultra-trace level analysis.
Manufacturing Advanced Materials
The production of materials like superalloys, specialized glass, and high-performance ceramics requires carefully controlled, high-temperature environments.
Crucibles provide the necessary vessel for these processes, such as in electron-beam melting, where material integrity and purity under extreme heat are paramount.
Why Material Selection is Critical
The material a crucible is made from directly dictates its use case. Choosing the wrong type can lead to contamination of the sample, or even catastrophic failure of the crucible itself.
Graphite Crucibles
Graphite is a common choice for its excellent thermal conductivity and high-temperature stability.
It is primarily used for the smelting of alloy tool steel and nonferrous metals like aluminum, brass, and copper.
Alumina Crucibles
Alumina (aluminum oxide) is a ceramic material known for its high strength and stability at very high temperatures.
These are frequently used in industrial settings for producing stainless steel and nickel alloys, as well as in other casting and molding processes where chemical inertness is vital.
Tantalum Crucibles
Tantalum is a high-performance metal prized for its immense strength, ductility, and exceptional resistance to both corrosion and extreme heat.
Because of these properties, tantalum crucibles often serve as a substitute for platinum in demanding laboratory work. They are critical in manufacturing superalloys and in the glass and ceramic industries.
Understanding the Trade-offs and Best Practices
Using a crucible is not merely about heating it. Proper handling and procedure are essential to ensure safety, prevent damage, and maintain the purity of your work.
The Critical Risk of Contamination
Never use the same crucible for different types of metals without thorough cleaning, and ideally, dedicate specific crucibles to specific metals.
Residue from a previous melt can contaminate the new batch, ruining an alloy or corrupting a chemical analysis.
The Danger of Physical and Thermal Stress
Crucibles are strong but can be brittle. They should always be handled with properly fitting tongs to avoid dropping them or creating stress points that could lead to cracks.
Placing a cold crucible into a red-hot furnace can cause thermal shock and failure. Gradual heating and cooling are always recommended.
Preventing Damage During Use
After a melt, a crucible should be emptied completely. If metal solidifies inside, it will expand upon re-heating and can easily crack the crucible from within.
To prevent the crucible from bonding to the furnace floor, a thin plate of cardboard can be placed underneath it before heating; the cardboard simply burns away.
Making the Right Choice for Your Goal
Your specific objective determines the right tool for the job. Choosing a crucible is no different, as it forms the foundation of any high-temperature process.
- If your primary focus is general non-ferrous metal melting: Graphite is the reliable, industry-standard choice for its excellent performance and value.
- If your primary focus is high-purity lab analysis or working with corrosive materials: Tantalum is the premium option, offering superior resistance and inertness for the most demanding applications.
- If your primary focus is industrial production of steel or nickel alloys: Alumina provides the high-temperature stability and chemical inertness required for these specific processes.
Ultimately, selecting the correct crucible is the first and most critical step toward achieving accurate, pure, and successful high-temperature work.
Summary Table:
| Material | Primary Use Case | Key Properties |
|---|---|---|
| Graphite | Melting non-ferrous metals (e.g., aluminum, brass) | Excellent thermal conductivity, high-temperature stability |
| Alumina | Industrial production of steel/nickel alloys, lab analysis | High strength, chemical inertness at high temperatures |
| Tantalum | High-purity lab work, superalloys, corrosive materials | Superior corrosion/heat resistance, ductility, inertness |
Ready to select the perfect crucible for your high-temperature process?
KINTEK specializes in high-performance lab equipment and consumables. Our experts can help you choose the right crucible—whether for metal melting, chemical analysis, or advanced material production—ensuring purity, safety, and optimal results for your laboratory.
Contact our team today for a consultation and elevate your lab's capabilities!
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- What is the role of a blast furnace or crucible melting furnace? Achieve Precise Aluminum Alloy Preparation
- What are the disadvantages of crucible furnace? Understanding the Trade-offs in Metal Melting
- Which crucible container can withstand high temperature and is used for metal and glass? Find the Right Material for Your Process
- How do I choose a crucible? Match Material, Temperature, and Application for Success
- What is the maximum temperature for clay crucibles? Find the Right Crucible for Your Melting Needs
- Why are magnesia crucibles selected for FeCrAl smelting? Ensure Purity & Stability in High-Temp Metal Casting
- What is a porcelain crucible? Your Essential Guide to High-Temp Lab Work
- Why are high-purity Alumina crucibles selected for molten salt corrosion? Ensure Data Accuracy with Inert Containers



















