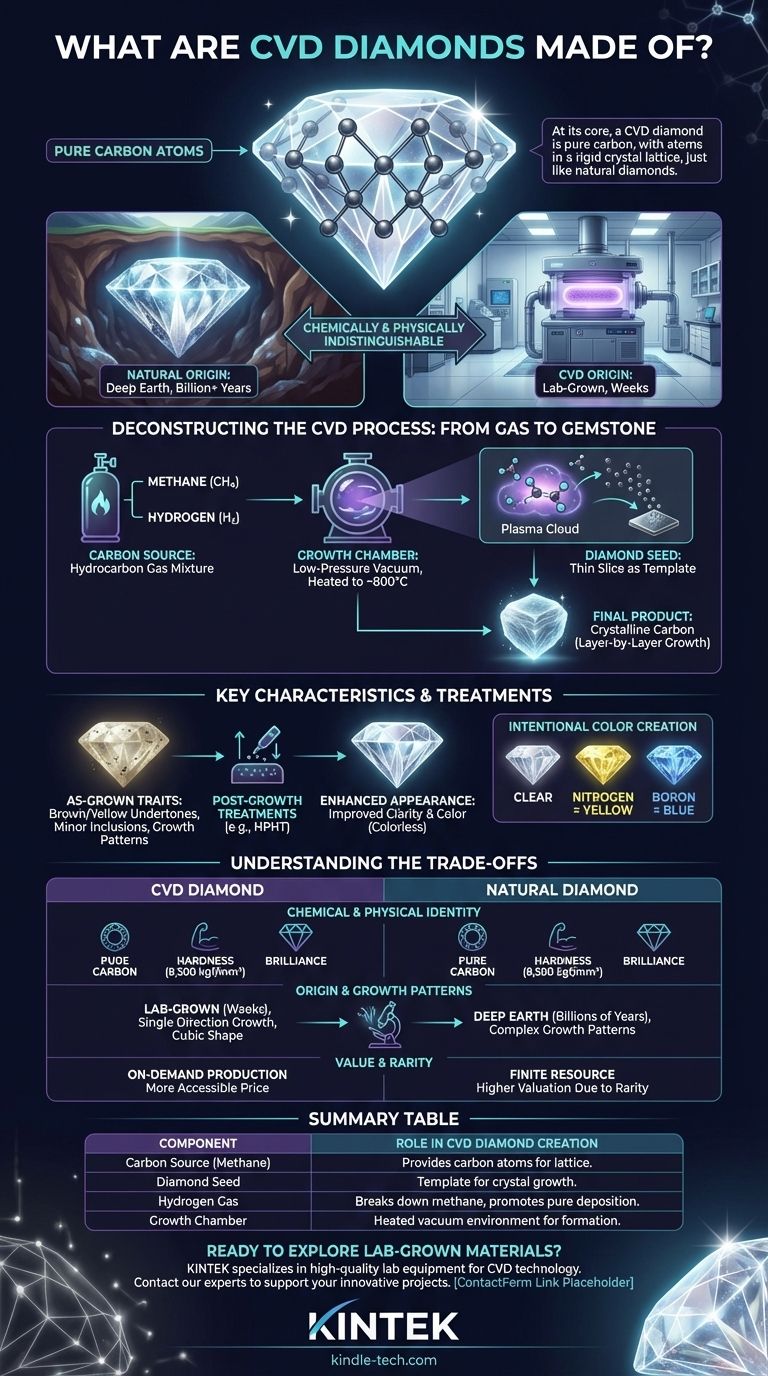
At its core, a CVD diamond is made of pure carbon. Just like a diamond mined from the earth, its atoms are arranged in a rigid crystal lattice structure. The critical difference is not its chemical composition but its origin—CVD diamonds are grown in a laboratory using a process that starts with a mixture of hydrocarbon gases.
The essential takeaway is that while the manufacturing process uses gases like methane and hydrogen, the final product is not a synthetic imitation. It is a true diamond, chemically and physically indistinguishable from its natural counterpart.

Deconstructing the CVD Process: From Gas to Gemstone
The term CVD stands for Chemical Vapor Deposition. This process methodically builds a diamond, atom by atom, in a highly controlled environment. It transforms simple gases into one of the hardest and most brilliant materials known.
The Diamond "Seed"
The process begins with a "seed," which is a very thin, flat slice of a previously grown diamond. This seed acts as the foundational template upon which the new diamond crystal will grow.
The Carbon Source
This diamond seed is placed inside a sealed, low-pressure vacuum chamber. A specific mixture of gases, typically methane (a hydrocarbon, CH4) and hydrogen, is introduced into this chamber.
The Growth Chamber
The chamber is heated to extreme temperatures, often around 800°C. This intense energy breaks the molecular bonds in the gas mixture, separating the carbon atoms from the hydrogen atoms.
The Final Product: Crystalline Carbon
These freed carbon atoms then "deposit" onto the surface of the diamond seed. Layer by layer, they bond with the seed's existing crystal structure, perfectly replicating it. The result is a larger, rough diamond composed of pure carbon.
Key Characteristics of CVD Diamonds
While chemically identical to natural diamonds, the unique growth process can result in specific characteristics that gemologists can identify.
Common Visual Traits
As-grown CVD diamonds can sometimes exhibit a brownish or yellowish undertone. They may also contain minor internal imperfections, such as dark spotty inclusions or visible graining patterns that reflect their layer-by-layer growth.
The Role of Post-Growth Treatments
To improve clarity and color, most CVD diamonds undergo a post-growth treatment process, such as HPHT (High-Pressure, High-Temperature). This treatment can significantly enhance a stone's appearance, making it colorless. However, it can occasionally make the diamond appear slightly milky or hazy.
Intentional Color Creation
The CVD process allows for precise control over the final product. By introducing specific trace elements during growth, manufacturers can create colored diamonds. Adding nitrogen creates yellow diamonds, while adding boron results in blue diamonds.
Understanding the Trade-offs
The choice between a CVD and a natural diamond is not about "real vs. fake" but about understanding their different origins and what that implies.
Chemical and Physical Identity
On a molecular level, they are the same. A CVD diamond has the same hardness (8,500 kgf/mm2), brilliance, and chemical makeup as a diamond formed deep within the Earth's mantle. They are indistinguishable to the naked eye.
Origin and Growth Patterns
A natural diamond forms over billions of years under immense geological pressure. In contrast, a CVD diamond grows in a lab over a matter of weeks. This results in different crystal growth patterns; CVD diamonds grow in a single direction with a cubic shape, which can sometimes cause internal strain visible only under high magnification.
Value and Rarity
The primary difference lies in rarity and perception. Natural diamonds are finite resources, which is a key factor in their valuation. Lab-grown diamonds can be produced on demand, which generally makes them a more accessible option.
Making the Right Choice for Your Goal
Your decision should be based on a clear understanding of what you value most in a gemstone.
- If your primary focus is geological origin and inherent rarity: A natural diamond, with its billion-year history, is the definitive choice.
- If your primary focus is maximizing size and quality for a given budget: A CVD diamond delivers identical chemical and visual properties, often at a more accessible price point.
- If your primary focus is a specific, vivid color: The controlled CVD process allows for the creation of intensely colored diamonds that are exceptionally rare and valuable in nature.
Ultimately, understanding that a CVD diamond is chemically pure carbon empowers you to make an informed choice based on your personal priorities.
Summary Table:
| Component | Role in CVD Diamond Creation |
|---|---|
| Carbon Source (Methane) | Provides the carbon atoms that build the diamond crystal lattice. |
| Diamond Seed | A thin diamond slice that acts as a template for crystal growth. |
| Hydrogen Gas | Helps break down methane and promotes pure carbon deposition. |
| Growth Chamber | A sealed vacuum chamber heated to ~800°C where the diamond forms. |
Ready to explore the potential of lab-grown materials in your research or production? KINTEK specializes in providing high-quality laboratory equipment and consumables for advanced material synthesis, including CVD technology. Whether you're developing new materials or optimizing your processes, our expertise can help you achieve precise, reliable results. Contact our experts today to discuss how we can support your innovative projects with the right tools and solutions.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- CVD Diamond for Thermal Management Applications
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
People Also Ask
- What is the effect of temperature on graphene oxide? Master Thermal Reduction for Precise Material Properties
- What is the mechanism of sputtering? A Guide to Precision Thin-Film Deposition
- What are the categories of carbon nanotubes? Understand SWCNT vs. MWCNT for Your Application
- What are the advantages of co sputtering? Engineer Custom Materials with Precise Composition Control
- What can carbon nanotubes replace? Upgrade Your Materials with Superior Performance
- What is the process of sputtering chemically? Master Reactive Sputtering for Superior Thin Films
- Can carbon nanotubes replace silicon? The Future of Computing Beyond Moore's Law
- What is the substrate material for thin film deposition? A Guide to Selecting the Right Foundation



















