At its core, the Lead Dioxide-Titanium Oxygen Evolution Electrode is a specialized tool for processes demanding powerful oxidation. Its primary applications span advanced wastewater treatment for destroying persistent pollutants and specific industrial electrosynthesis, where its high potential is necessary to drive desired chemical reactions.
This electrode's value lies in its exceptionally high oxygen evolution potential. This property enables it to generate powerful oxidizing agents capable of breaking down complex molecules that are resistant to other treatment methods.
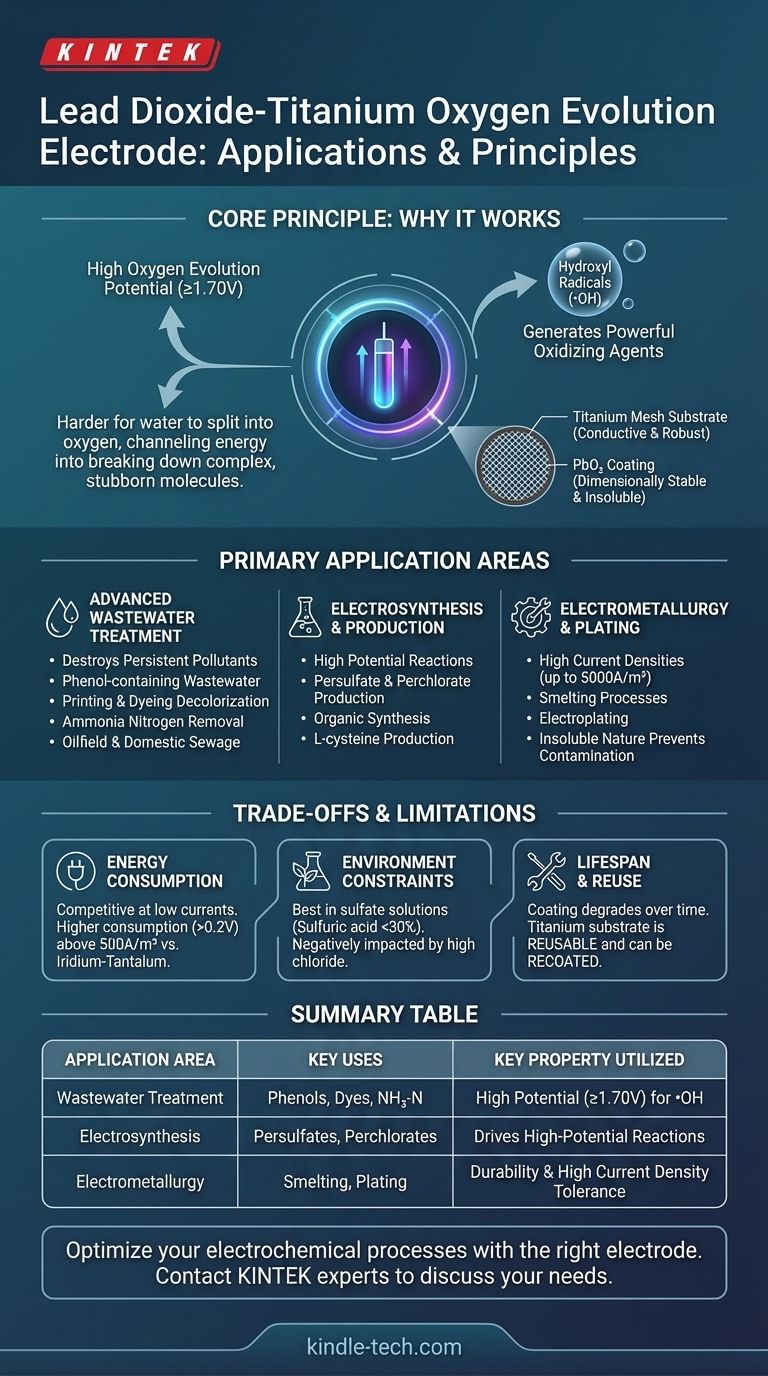
The Core Principle: Why It Works
The effectiveness of this electrode is not accidental; it stems from a unique combination of electrochemical properties and physical design. Understanding these principles is key to deploying it correctly.
High Oxygen Evolution Potential
The defining characteristic is its high oxygen evolution potential, which is ≥ 1.70V. In simple terms, it makes it "harder" for water to split into oxygen gas on the electrode's surface.
This extra energy doesn't go to waste. Instead, it is channeled into creating highly reactive and powerful oxidizing species, most notably hydroxyl radicals (•OH).
Strong Oxidizing Power
The generation of hydroxyl radicals is the true engine behind this electrode's performance in treatment applications. These radicals are extremely effective at breaking down complex and stubborn organic pollutants into simpler, less harmful substances like CO₂ and water.
This makes it invaluable for treating industrial wastewater containing phenols, dyes, and other persistent organic compounds.
Robust and Insoluble Design
The electrode is built on a titanium mesh substrate, which provides structural integrity and good conductivity. This base is coated with lead dioxide (PbO₂).
This construction results in a dimensionally stable, or insoluble, anode. It resists corrosion and does not readily dissolve during operation, ensuring a long service life in demanding industrial environments.
Primary Application Areas
The electrode's unique properties make it suitable for a range of demanding inorganic and organic processes. These can be grouped into a few key categories.
Advanced Wastewater Treatment
This is the most common and critical application area. Its ability to destroy persistent pollutants makes it a powerful tool for environmental remediation.
Specific uses include the treatment of:
- Phenol-containing wastewater
- Printing and dyeing wastewater (decolorization)
- Oilfield and oily wastewater
- Ammonia nitrogen wastewater
- General domestic sewage (when complex contaminants are present)
Electrosynthesis and Production
The high potential enables the creation of chemicals that are difficult to synthesize through other means. The electrode provides the necessary electrochemical "force" to drive these reactions.
Key production processes include:
- Persulfate and Perchlorate Production: Creating highly oxidized salts.
- Organic Synthesis: Facilitating reactions requiring a high oxidation potential.
- L-cysteine Production: An example of its use in biochemical synthesis.
Electrometallurgy and Plating
In fields like smelting and electroplating, the electrode is valued for its durability and ability to operate at high current densities (up to 5000A/m²). Its insoluble nature prevents contamination of the plating bath or smelted metal.
Understanding the Trade-offs and Limitations
No single solution is perfect for every scenario. While powerful, this electrode has specific operational trade-offs that must be considered.
Energy Consumption
At low current densities, its energy consumption is competitive with other common anodes like iridium-tantalum.
However, at current densities above 500A/m², its energy consumption is approximately 0.2V higher than an iridium-tantalum anode. This can lead to increased operational costs in high-intensity applications.
Chemical Environment Constraints
This electrode performs best in specific chemical environments. It is highly effective in solutions containing sulfate (SO₄²⁻) and is specified for use in sulfuric acid concentrations below 30%.
Its performance can be negatively impacted in environments with high concentrations of other ions, particularly chloride (Cl⁻), which may require a different type of anode.
Coating Lifespan and Substrate Reuse
While the PbO₂ coating is robust, it will eventually degrade over its service life. The lifespan is dependent on the current density, solution chemistry, and operating temperature.
A key advantage is that the titanium substrate is reusable. Once the coating is compromised, the electrode can be stripped and recoated, reducing long-term replacement costs.
Making the Right Choice for Your Process
Selecting the correct anode requires balancing performance needs with operational costs. Your primary goal will dictate the best choice.
- If your primary focus is treating highly persistent organic pollutants: The Lead Dioxide-Titanium electrode's superior oxidizing power makes it the most effective choice.
- If your primary focus is maximizing energy efficiency at high currents (>500A/m²): An Iridium-Tantalum anode might offer a lower operational cost due to its lower voltage requirement.
- If your primary focus is long-term stability in a sulfate-rich environment: This electrode is an excellent and cost-effective option, especially with its potential for recoating.
Ultimately, choosing the right electrode is about matching its specific strengths to the unique chemical and economic demands of your application.
Summary Table:
| Application Area | Key Uses | Key Property Utilized |
|---|---|---|
| Advanced Wastewater Treatment | Destroying phenols, dyes, ammonia nitrogen | High oxygen evolution potential (≥1.70V) for generating hydroxyl radicals |
| Electrosynthesis & Production | Producing persulfates, perchlorates, L-cysteine | Ability to drive high-potential chemical reactions |
| Electrometallurgy & Plating | Smelting, electroplating processes | Durability & high current density tolerance (up to 5000A/m²) |
Optimize your electrochemical processes with the right electrode.
KINTEK specializes in high-performance lab equipment and consumables, including advanced electrodes for industrial and research applications. Our expertise can help you select the ideal solution to enhance your wastewater treatment efficiency, electrosynthesis yields, or plating quality.
Contact our experts today to discuss your specific needs and discover how our solutions can drive your success.
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Iridium Dioxide IrO2 for Water Electrolysis
People Also Ask
- What is the RRDE in electrochemistry? Unlock Detailed Reaction Pathways with Dual-Electrode Analysis
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- What is the difference between ring disk electrode and rotating disk electrode? Unlock Deeper Electrochemical Insights
- What is the difference between RDE and RRDE? Unlock Advanced Electrochemical Reaction Analysis


















