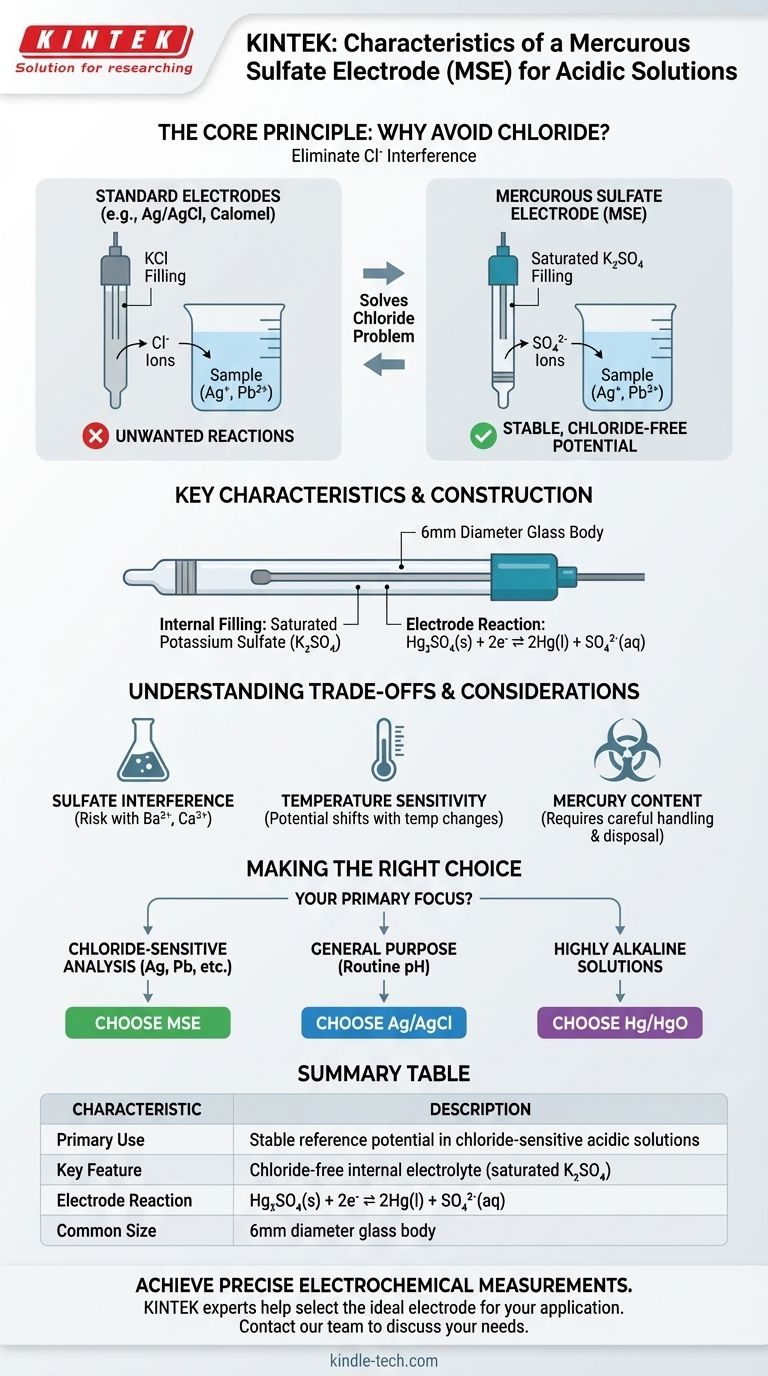
A mercurous sulfate electrode (MSE) designed for acidic solutions is characterized by its specific internal electrolyte and physical construction. It typically has a diameter of 6mm and is filled with a saturated potassium sulfate (K₂SO₄) solution, making it a specialized tool for chloride-sensitive electrochemical measurements.
The core purpose of a mercurous sulfate electrode is to provide a stable reference potential in environments where the chloride ions from more common electrodes (like Ag/AgCl or Calomel) would interfere with the measurement.

The Core Principle: Why Avoid Chloride?
The primary reason to choose a mercurous sulfate electrode stems from the need to eliminate chloride ions (Cl⁻) from the electrochemical system. This is a critical requirement in many specific analytical scenarios.
The Problem with Standard Electrodes
Most common reference electrodes, such as Silver/Silver Chloride (Ag/AgCl) and Saturated Calomel Electrodes (SCE), are filled with a concentrated potassium chloride (KCl) solution. This is by design, as the constant chloride concentration provides a stable reference potential.
Unwanted Reactions and Interferences
In many experiments, chloride ions are an active participant, not a passive observer. A small amount of the KCl filling solution will inevitably leak from the electrode's junction into the sample solution. This can cause significant errors if the sample contains ions that precipitate with chloride, such as silver (Ag⁺), lead (Pb²⁺), or mercury(I) (Hg₂²⁺).
The Chloride-Free Alternative
The mercurous sulfate electrode solves this problem directly. By using a non-interfering sulfate-based electrolyte (K₂SO₄), it provides a stable reference potential without introducing problematic chloride ions into the sample.
Key Characteristics and Construction
The design of the MSE is optimized for its specific function as a chloride-free reference.
The Internal Filling Solution
The electrode is filled with a saturated potassium sulfate (K₂SO₄) solution. The saturation ensures a constant and well-defined activity of sulfate ions (SO₄²⁻) at a given temperature, which is essential for a stable and reproducible potential.
The Electrode Reaction
The potential of the MSE is based on the reversible electrochemical reaction involving mercury and mercurous sulfate:
Hg₂SO₄(s) + 2e⁻ ⇌ 2Hg(l) + SO₄²⁻(aq)
This equilibrium provides the stable voltage against which other potentials in the cell are measured.
Physical Design
As noted in reference materials, a common form factor for these electrodes is a 6mm diameter glass body. This standard size makes it compatible with most laboratory electrochemical cells and apparatus.
Understanding the Trade-offs
While invaluable, the mercurous sulfate electrode is not a universal solution. Its use involves specific considerations and potential downsides.
Potential for Sulfate Interference
Just as the MSE solves the chloride interference problem, it introduces sulfate ions. In experiments where sulfate ions could react with sample components (such as barium, calcium, or strontium), this electrode would be an unsuitable choice.
Temperature Sensitivity
Like all electrodes that use a saturated salt solution, the potential of the MSE is sensitive to changes in temperature. This is because the solubility of K₂SO₄ changes with temperature, altering the concentration of sulfate ions and thus shifting the electrode's reference potential.
Environmental and Handling Concerns
The electrode contains mercury, a toxic heavy metal. This requires careful handling, storage, and disposal procedures in accordance with laboratory safety and environmental regulations.
Making the Right Choice for Your Application
Selecting the correct reference electrode is not about finding the "best" one, but the most appropriate one for your specific analytical goal.
- If your primary focus is analyzing solutions containing silver, lead, or other ions that precipitate with chloride, the mercurous sulfate electrode is the correct choice.
- If your primary focus is general-purpose measurements (like routine pH testing) in samples without chloride sensitivity, a more common and robust Ag/AgCl electrode is often preferable.
- If your primary focus is working in highly alkaline (basic) solutions, neither of the above is ideal; a dedicated electrode like a mercuric oxide (Hg/HgO) electrode should be used instead.
Choosing the right reference electrode is foundational to achieving accurate and reliable electrochemical measurements.
Summary Table:
| Characteristic | Description |
|---|---|
| Primary Use | Stable reference potential in chloride-sensitive acidic solutions |
| Key Feature | Chloride-free internal electrolyte (saturated K₂SO₄) |
| Electrode Reaction | Hg₂SO₄(s) + 2e⁻ ⇌ 2Hg(l) + SO₄²⁻(aq) |
| Common Size | 6mm diameter glass body |
| Considerations | Avoids chloride interference; potential sulfate interference; temperature sensitivity; contains mercury |
Achieve precise electrochemical measurements in chloride-sensitive environments with the right reference electrode. KINTEK specializes in laboratory equipment and consumables, providing reliable solutions for your analytical challenges. Our experts can help you select the ideal electrode for your specific application. Contact our team today to discuss your needs and ensure the accuracy of your results.
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Copper Sulfate Reference Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
People Also Ask
- What are the characteristics of a saturated calomel electrode for neutral solutions? Understanding its stability and limitations.
- Why is the calomel electrode used as a secondary reference electrode? A Practical Guide to Stable Measurements
- Which electrode is used as a reference? A Guide to Accurate Electrochemical Measurements
- Which type of electrode can be used as a reference point? Select the Right One for Accurate Measurements
- Why is a Saturated Calomel Electrode (SCE) used as a reference electrode in microbial fuel cell research?



















