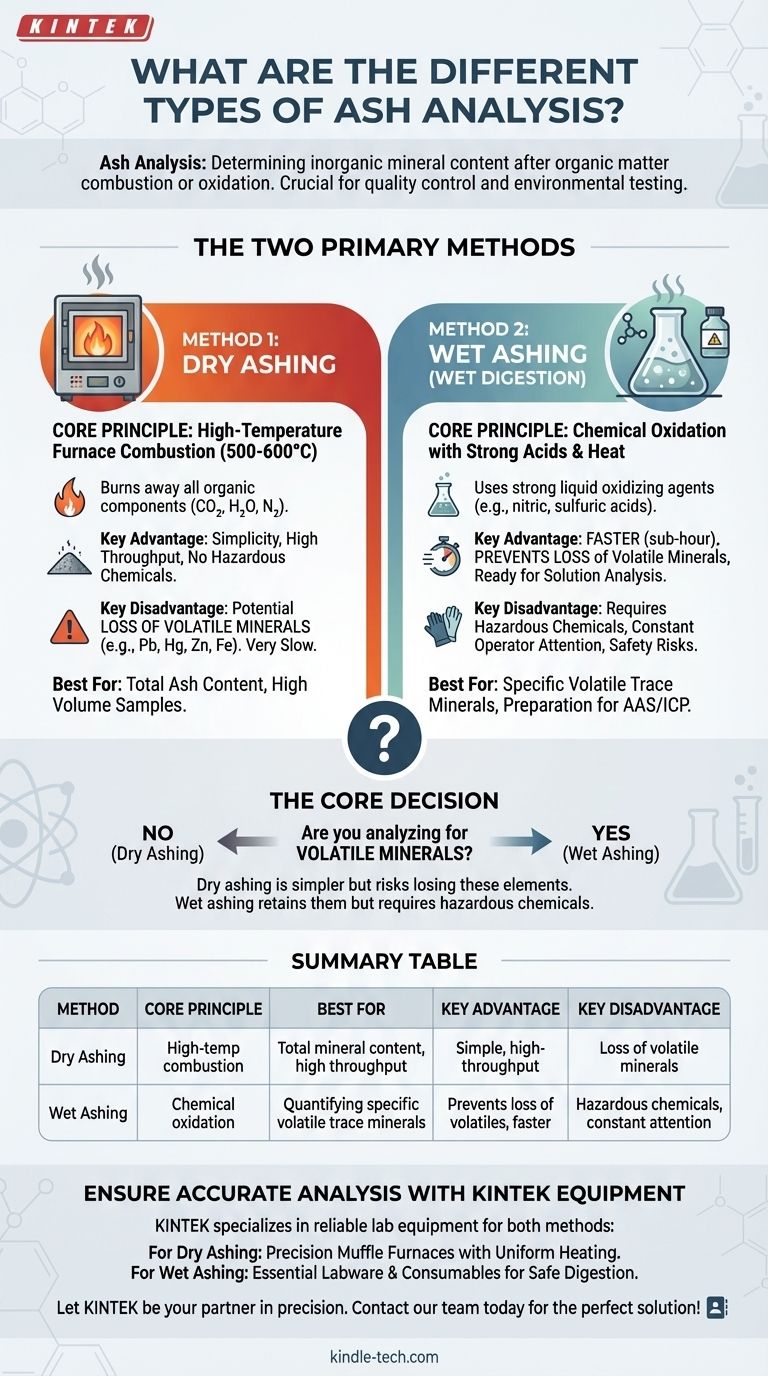
The two primary methods for ash analysis are dry ashing and wet ashing (also known as wet digestion). Dry ashing uses high-temperature furnace combustion to remove all organic material, while wet ashing uses strong acids and heat to achieve the same goal. The choice between them is dictated by the specific minerals you need to measure and the characteristics of your sample.
The core decision between dry and wet ashing hinges on a single question: Are you analyzing for volatile minerals? Dry ashing is simpler but risks losing these elements, whereas wet ashing retains them but requires handling hazardous chemicals.

What is Ash Analysis?
Ash is the inorganic residue that remains after the complete combustion or oxidation of organic matter. Ash analysis is a crucial technique used to determine the total mineral content in a sample.
The Purpose of Ashing
This analysis is not just a single measurement but a preparatory step for further analysis. It is essential for quality control in food science, nutritional labeling, and environmental testing to quantify the essential mineral and toxic heavy metal content of a substance.
Method 1: Dry Ashing
Dry ashing is the most common method for determining total ash content. It relies on the principle of high-temperature incineration to burn away all organic components.
The Core Principle
A sample is placed in a high-temperature muffle furnace, typically between 500 and 600°C. The extreme heat causes all organic substances to oxidize and turn into gases (CO₂, H₂O, N₂), leaving only the non-combustible inorganic minerals behind.
Key Advantages
The primary benefits of dry ashing are its simplicity and high throughput. An operator can place many samples into a furnace at once and leave them to process with minimal supervision. It also avoids the use of harsh or dangerous chemical reagents.
Critical Disadvantages
The main drawback is the potential for significant loss of volatile minerals. Elements like lead, zinc, iron, and mercury can vaporize and be lost at the high temperatures used. The process is also very slow, often taking several hours or even overnight to complete.
Method 2: Wet Ashing (Wet Digestion)
Wet ashing, or wet digestion, is a chemical approach to destroying the organic matrix. It is the preferred method when analyzing for specific trace minerals that might be lost during dry ashing.
The Core Principle
Instead of heat alone, this method uses strong liquid oxidizing agents—typically a combination of acids like nitric acid, sulfuric acid, or perchloric acid—along with heat. These chemicals break down and dissolve the organic matter, leaving the minerals suspended in an acidic solution.
Key Advantages
Wet digestion is much faster than dry ashing, usually taking less than an hour. Crucially, it operates at much lower temperatures, which prevents the loss of volatile minerals. The resulting sample is already in solution, making it ideal for subsequent analysis with techniques like Atomic Absorption Spectroscopy (AAS).
Critical Disadvantages
This method requires constant operator attention and can only be performed on a small number of samples at a time. The primary concern is safety, as it involves handling highly corrosive and hazardous acids, requiring the use of a specialized fume hood.
Understanding the Trade-offs
Choosing the correct method requires a clear understanding of the compromises you are making between speed, safety, and analytical accuracy.
Volatility vs. Simplicity
Dry ashing offers operational simplicity but at the cost of potentially inaccurate results for volatile elements. If your goal is simply "total ash," it is often sufficient. If your goal is quantifying specific trace metals, its simplicity is a liability.
Speed vs. Throughput
Wet ashing is significantly faster for a single sample. However, you can only process a few samples at once. Dry ashing is very slow, but a large furnace can process dozens or even hundreds of samples simultaneously, making its total throughput much higher for large batches.
Safety and Equipment
The safety risks are fundamentally different. Dry ashing involves the hazard of an extremely hot furnace. Wet ashing involves the more complex chemical hazard of working with fuming, corrosive acids, which mandates specialized ventilation and personal protective equipment.
Making the Right Choice for Your Analysis
Your analytical goal should be the sole determinant of the method you choose.
- If your primary focus is total mineral content for quality control or labeling: Dry ashing is the simpler, safer, and higher-throughput choice, assuming no volatile minerals of interest.
- If your primary focus is quantifying specific volatile trace minerals (e.g., lead, mercury, zinc): Wet digestion is the only reliable method to ensure these elements are not lost.
- If your primary focus is preparing a sample for analysis by AAS or ICP: Wet digestion is often more efficient as it leaves the minerals in a liquid solution ready for direct analysis.
Ultimately, selecting the correct ashing technique is the first step in guaranteeing the accuracy and relevance of your mineral analysis results.
Summary Table:
| Method | Core Principle | Best For | Key Advantage | Key Disadvantage |
|---|---|---|---|---|
| Dry Ashing | High-temperature furnace combustion (500-600°C) | Total mineral content, high sample throughput | Simple, high-throughput, no hazardous chemicals | Potential loss of volatile minerals (e.g., Pb, Hg, Zn) |
| Wet Ashing | Chemical oxidation with strong acids and heat | Quantifying specific volatile trace minerals | Prevents loss of volatile elements, faster for single samples | Requires hazardous chemicals, constant operator attention |
Ensure Accurate Mineral Analysis with the Right Equipment
Choosing the correct ashing method is critical for your lab's results. KINTEK specializes in providing the reliable lab equipment you need to perform both dry and wet ashing with confidence.
- For Dry Ashing: Our range of high-temperature muffle furnaces offers precise temperature control and uniform heating for consistent, high-throughput ash content determination.
- For Wet Ashing: We supply the essential labware and consumables required for safe acid digestion, helping you handle hazardous materials securely.
Let KINTEK be your partner in precision. Our experts can help you select the ideal equipment for your specific application, ensuring safety, efficiency, and accurate results for your mineral analysis.
Contact our team today to discuss your lab's needs and find the perfect solution!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the difference between muffle furnace and air oven? Choose the Right Tool for Your Thermal Process
- How do you prepare samples for IR? A Guide to Solid, Liquid, and Gas Sample Prep
- Which metals cannot be hardened by heat treatment? Understand the limits of thermal hardening.
- What are the safety precautions during heat treatment process? A Guide to Mitigating Thermal, Atmospheric, and Mechanical Hazards
- How do you control a muffle furnace? Master Precise Temperature Control for Your Lab



















