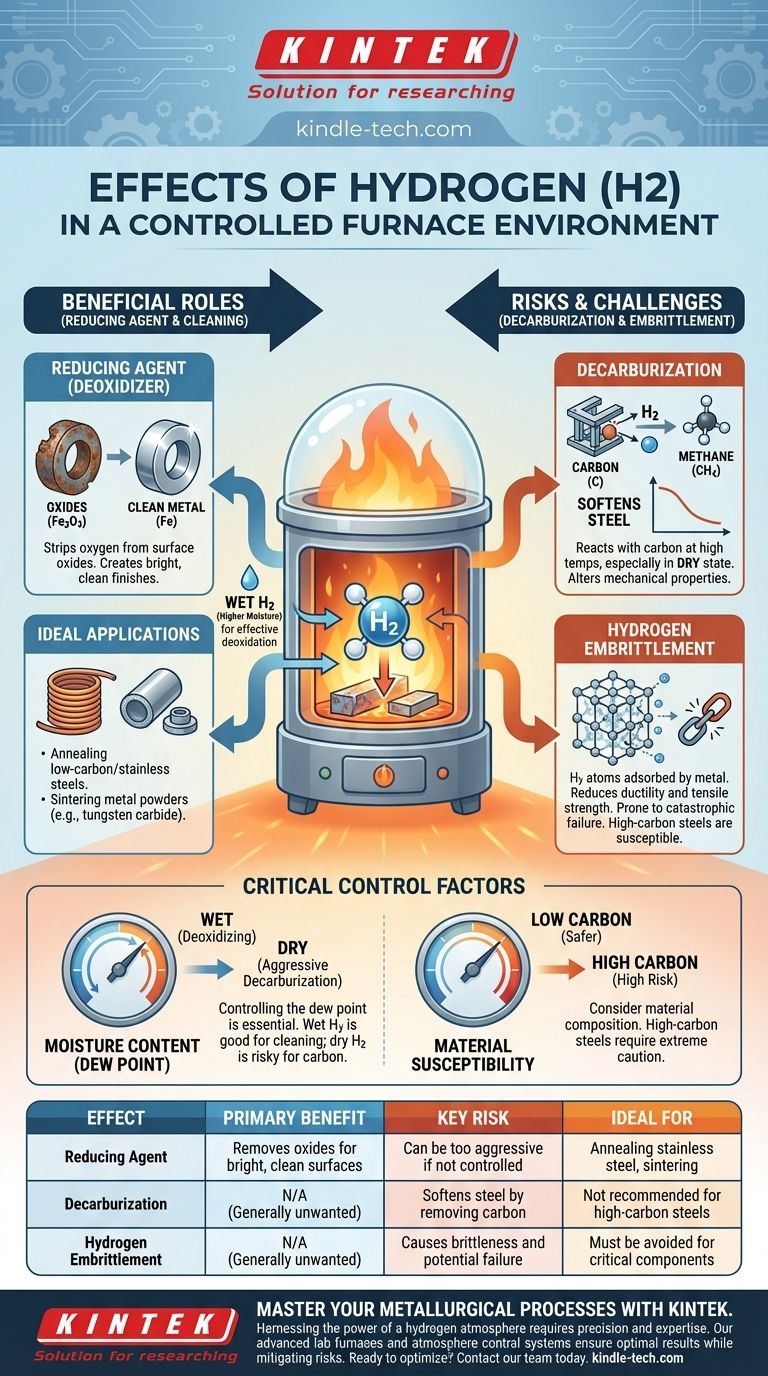
In a controlled furnace, hydrogen (H2) acts as a powerful chemical agent, primarily used to reduce metal oxides and remove impurities from material surfaces. It is highly effective at creating clean, bright finishes but also introduces significant risks, including the unwanted removal of carbon from steel (decarburization) and a dangerous phenomenon known as hydrogen embrittlement.
The core effect of hydrogen is its intense reactivity at high temperatures. This reactivity can be harnessed for beneficial cleaning and reduction, but it must be meticulously controlled to avoid damaging the material's structural integrity.

The Primary Roles of Hydrogen in Furnace Atmospheres
Hydrogen is not an inert background gas; it is an active participant in high-temperature metallurgical processes. Its behavior is dictated by temperature, moisture content, and the material being treated.
A Powerful Reducing Agent
The most common use for a hydrogen atmosphere is to act as a reducing agent, or deoxidizer. It chemically strips oxygen from metal oxides that form on the surface of parts.
For example, hydrogen reacts with iron oxide (rust) to form pure iron and water vapor. This leaves the material exceptionally clean and bright, a critical requirement for processes like annealing and sintering.
The Decarburization Effect
At very high temperatures, particularly in a dry state, hydrogen can react with the carbon present within steel.
This reaction forms methane (CH4), effectively pulling carbon out of the steel's surface. This decarburization can be detrimental, as it softens the steel and alters its intended mechanical properties.
Understanding the Trade-offs and Risks
Using hydrogen successfully means managing its dual nature. The same chemical properties that make it a superb cleaning agent also make it potentially destructive.
The Critical Role of Moisture
The effectiveness and behavior of hydrogen are critically limited by its moisture content.
A "wet" hydrogen atmosphere (with higher moisture) is excellent for deoxidation. Conversely, a very "dry" hydrogen atmosphere is a much more aggressive decarburizing agent. Controlling the dew point is therefore essential.
The Danger of Hydrogen Embrittlement
Hydrogen embrittlement occurs when individual hydrogen atoms are adsorbed by the metal, migrating into its crystalline structure.
This process significantly reduces the material's ductility and tensile strength, making it brittle and prone to catastrophic failure under stress. High-carbon substances are particularly susceptible to this risk.
Purity and System Design
Commercial hydrogen is typically 98% to 99.9% pure, with trace impurities like water vapor, oxygen, and nitrogen. These impurities must be managed.
To ensure safety and process integrity, furnaces often use a sealed inner chamber, or retort, to contain the hydrogen atmosphere. This protects the furnace heating elements from chemical attack and contains any potentially hazardous compounds.
How to Apply This to Your Process
Choosing to use a hydrogen atmosphere depends entirely on your material and your desired outcome.
- If your primary focus is annealing low-carbon steels or stainless steels: A hydrogen atmosphere is highly effective for reducing surface oxides to achieve a bright, clean finish.
- If your primary focus is processing high-carbon steels: You must proceed with extreme caution, as the risks of unwanted decarburization and hydrogen embrittlement are very high.
- If your primary focus is sintering metal powders (e.g., tungsten carbide): A dry hydrogen atmosphere is ideal for removing residual oxides, which promotes superior bonding between the powder particles.
Ultimately, mastering a hydrogen furnace atmosphere means treating it not as a simple environment, but as a precise metallurgical tool.
Summary Table:
| Effect of Hydrogen | Primary Benefit | Key Risk | Ideal For |
|---|---|---|---|
| Reducing Agent | Removes oxides for bright, clean surfaces | Can be too aggressive if not controlled | Annealing stainless steel, sintering |
| Decarburization | N/A (Generally unwanted) | Softens steel by removing carbon | Not recommended for high-carbon steels |
| Hydrogen Embrittlement | N/A (Generally unwanted) | Causes brittleness and potential failure | Must be avoided for critical components |
Master Your Metallurgical Processes with KINTEK
Harnessing the power of a hydrogen furnace atmosphere requires precision and expertise. Whether you are annealing for a bright finish or sintering metal powders, the right equipment is critical to achieving your desired material properties while mitigating risks like decarburization and embrittlement.
KINTEK specializes in advanced lab furnaces and atmosphere control systems designed for safety and performance. Our experts can help you select the perfect solution for your specific application, ensuring optimal results for your laboratory's unique needs.
Ready to optimize your high-temperature processes? Contact our team today to discuss how our solutions can bring precision and reliability to your lab.
Visual Guide
Related Products
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vertical Laboratory Tube Furnace
People Also Ask
- Why are high-vacuum or atmospheric high-temperature furnaces required for phosphate glass matrices? Expert Guide
- What sintering temperatures may be required for tungsten in a pure hydrogen atmosphere? Reach 1600°C for Peak Performance
- What role does an atmosphere furnace utilizing hydrogen gas play in Cu-Cr-Nb alloy powder pretreatment? (Key Insights)
- What is a hydrogen oven? The Future of Clean, High-Temperature Cooking
- How does a high-temperature atmosphere furnace simulate service environments for evaluating CMAS corrosion resistance?
- What roles does an atmosphere tube furnace play in FeAl/Al2O3/TiO2 coating? Expert Guide to Advanced Layer Synthesis
- What is a nitrogen oven? Essential Guide to Oxidation-Free Thermal Processing
- Why is a high-temperature atmosphere furnace required for 70-hour alloy annealing? Achieve Material Homogenization



















