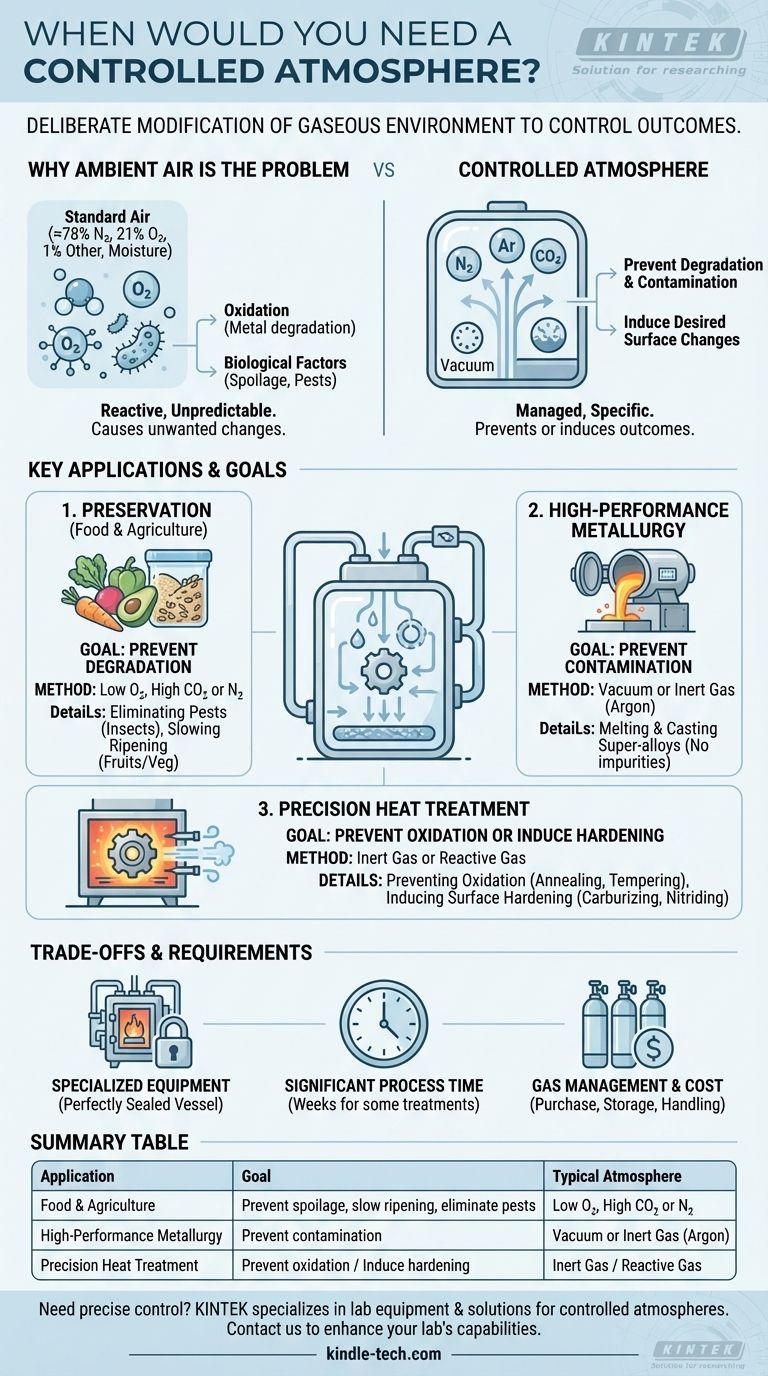
In short, a controlled atmosphere is necessary whenever the standard ambient air would interfere with, contaminate, or prevent a desired outcome. It is used in applications ranging from large-scale food preservation to the highly precise manufacturing of advanced metal alloys and heat treatment processes. The core principle is the deliberate modification of the gaseous environment to control chemical reactions and biological processes.
The decision to use a controlled atmosphere is driven by a need for absolute control. It's about replacing the unpredictable, reactive nature of normal air with a specific, managed environment to either prevent unwanted changes like oxidation and spoilage or to intentionally induce desired chemical changes on a material's surface.

Why Ambient Air Is Often the Problem
To understand the need for a controlled atmosphere, you first have to recognize the issues with using standard air. Ambient air is approximately 78% nitrogen, 21% oxygen, and 1% other gases, along with variable moisture.
The Impact of Oxygen
Oxygen is highly reactive, especially at elevated temperatures. This reactivity, known as oxidation, is a primary driver for using controlled atmospheres in metallurgy. It causes scaling and degrades the quality of metals during heat treatment.
The Impact of Biological Factors
Standard air allows aerobic organisms—from insects to microbes—to thrive. This is a critical problem in the storage of agricultural products, where pests can destroy entire harvests.
Key Applications for Controlled Atmospheres
Controlled atmospheres are not a single solution but a category of techniques applied across different industries. The specific gas mixture or the use of a vacuum is tailored to the goal.
1. Preservation of Food and Agriculture
The primary goal here is to prevent degradation. By altering the air within a sealed storage environment, you can dramatically extend the shelf life of perishable goods.
Eliminating Pests
For grains, legumes, and oilseeds, the main purpose is to control insect pests. Most insects require oxygen to survive, so replacing it with carbon dioxide or nitrogen creates an environment where they cannot live.
Slowing Ripening
This technique is also used for fresh produce like fruits and vegetables. Reducing oxygen and increasing carbon dioxide slows the natural respiration and ripening process, keeping the product fresh for much longer.
2. High-Performance Metallurgy
In the production of advanced materials, the goal is to prevent contamination. The slightest impurity can compromise the structural integrity of high-performance alloys.
Melting and Casting Super-alloys
Materials like nickel-based super-alloys, used in jet engines and turbines, must be perfect. They are melted and cast in a vacuum or an inert gas atmosphere (like argon) to prevent oxygen and nitrogen from dissolving into the metal and creating weak points.
3. Precision Heat Treatment
For many heat treatment processes, the atmosphere is not just a protective blanket but an active ingredient in a chemical reaction.
Preventing Oxidation (Annealing & Tempering)
When heating a metal to soften it (annealing) or temper it, an inert atmosphere prevents the formation of surface oxide scale. This results in a clean, "bright" finish, often called bright annealing.
Inducing Surface Hardening (Carburizing & Nitriding)
Conversely, some processes use a reactive atmosphere to change the material's properties. In carburizing, a carbon-rich atmosphere is used to force carbon atoms into the surface of steel, making it significantly harder. Nitriding uses a nitrogen-rich atmosphere to achieve a similar hardening effect.
Understanding the Trade-offs and Requirements
Implementing a controlled atmosphere is a significant technical undertaking with specific requirements. It is not a simple or inexpensive solution.
Specialized Equipment is Mandatory
You cannot achieve a controlled atmosphere without a perfectly sealed vessel. In industrial settings, this often involves specialized equipment like tube furnaces with sealed work tubes or chamber furnaces fitted with sealed retorts.
Process Time Can Be Significant
These treatments are not always fast. For example, treating grain to eliminate pests can take several weeks, especially at lower ambient temperatures.
Gas Management and Cost
The gases used (nitrogen, argon, carbon dioxide, hydrogen) have associated costs for purchase, storage, and handling. Maintaining the precise gas mixture required for a process demands sophisticated control and monitoring systems, adding to the operational complexity.
Making the Right Choice for Your Goal
The choice to use a controlled atmosphere—and which one to use—depends entirely on your final objective.
- If your primary focus is preservation: Your goal is to create an inhospitable environment for biological organisms by removing oxygen and/or increasing carbon dioxide.
- If your primary focus is purity: Your goal is to eliminate all reactive gases to prevent contamination, which typically requires a high vacuum or a high-purity inert gas like argon.
- If your primary focus is surface transformation: Your goal is to use a specific, reactive gas mixture as an ingredient to deliberately alter the chemical composition of your material's surface.
Ultimately, a controlled atmosphere is implemented when the environment itself is a critical variable in achieving success.
Summary Table:
| Application | Goal | Typical Atmosphere |
|---|---|---|
| Food & Agriculture Preservation | Prevent spoilage, slow ripening, eliminate pests | Low O₂, High CO₂ or N₂ |
| High-Performance Metallurgy | Prevent contamination during melting/casting | Vacuum or Inert Gas (Argon) |
| Precision Heat Treatment | Prevent oxidation or induce surface hardening | Inert Gas or Reactive Gas (for carburizing/nitriding) |
Need precise control over your process environment? KINTEK specializes in lab equipment and consumables, providing the reliable solutions you need for creating controlled atmospheres in your laboratory. Whether you're working with heat treatment furnaces, vacuum systems, or gas management, our expertise ensures your materials are protected from contamination and your processes achieve the desired outcomes. Contact us today to discuss how we can support your specific application and enhance your lab's capabilities.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is argon used instead of nitrogen? Ensuring Absolute Inertness for High-Stakes Applications
- Why is a high-purity Argon atmosphere essential for melting Uranium and Zirconium? Ensure Metal Integrity
- Why are protective atmospheres necessary in sintering? Prevent Oxidation for Stronger Parts
- Why is an atmosphere tube furnace necessary for carbon-coated silicon anodes? Ensure Peak Material Purity
- What is a chemically reducing atmosphere? A Guide to Oxidation-Free Environments
- What is the role of the furnace atmosphere? Master Precise Metallurgical Control for Your Heat Treatment
- What is carburizing in case hardening? Achieve Superior Wear Resistance and Core Toughness
- Why is an atmosphere control high-temperature furnace required for MoS2 & graphene? Achieve Peak Material Performance



















