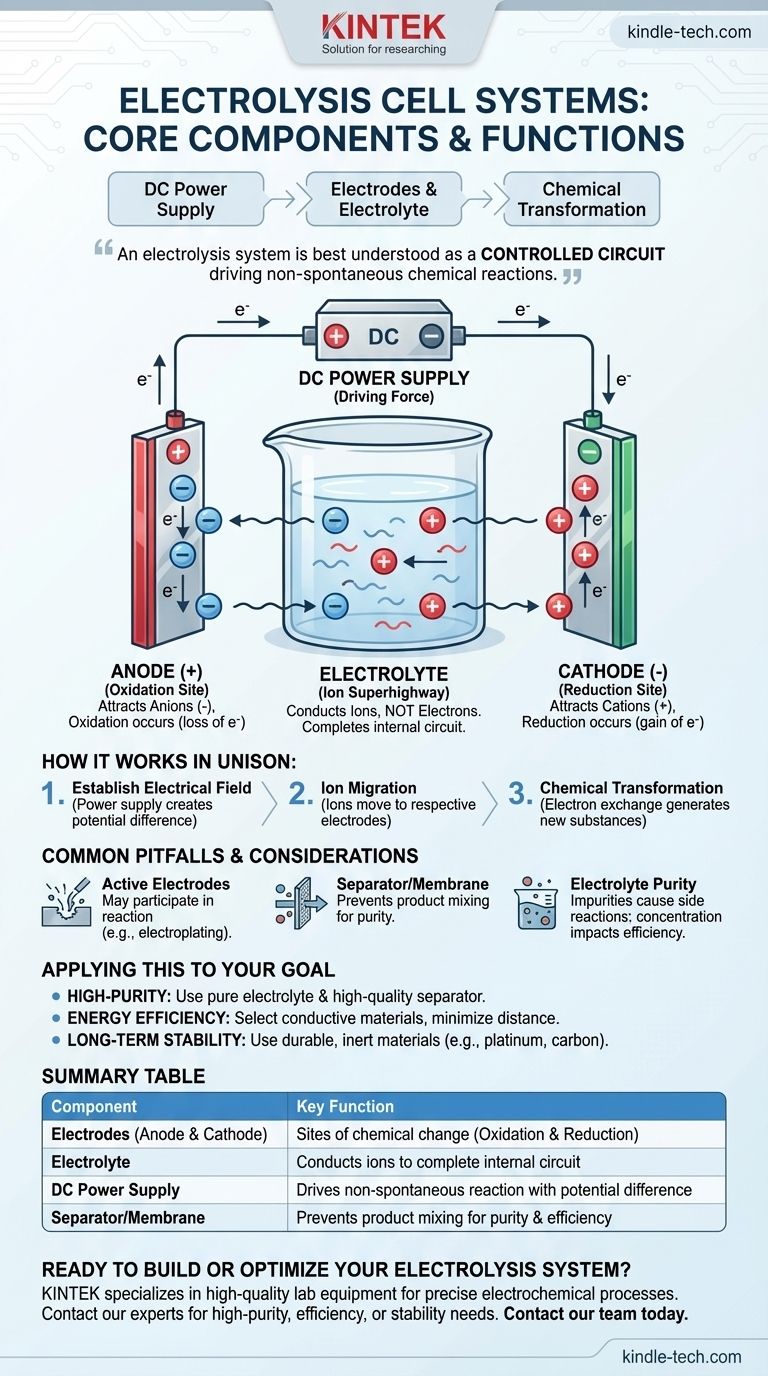
At its core, an electrolysis cell system is composed of three essential components: two electrodes (an anode and a cathode), an electrolyte containing ions, and an external DC power supply. These elements work in unison to use electrical energy to force a chemical reaction that would not happen on its own.
An electrolysis system is best understood not as a collection of parts, but as a controlled circuit. The power supply creates an electrical potential, driving ions through the electrolyte to the electrodes, where they undergo chemical transformations.
The Core Components and Their Roles
To grasp how electrolysis works, you must first understand the specific function of each primary component. Each one plays an indispensable and distinct role in the process.
The Electrodes: Sites of Chemical Change
The anode and cathode are conductive materials, typically metals or graphite, that serve as the physical interface between the external circuit and the electrolyte.
The anode is the positive electrode. It attracts negatively charged ions (anions) from the electrolyte. At the anode's surface, these ions lose electrons in a process called oxidation.
The cathode is the negative electrode. It attracts positively charged ions (cations). Here, the ions gain electrons in a process called reduction.
The Electrolyte: The Ion Superhighway
The electrolyte is a substance containing free-moving ions, which makes it electrically conductive. It is often a solution of an acid, base, or salt dissolved in water.
Its sole purpose is to conduct ions, not electrons. The movement of these ions between the electrodes completes the electrical circuit inside the cell, allowing the reaction to be sustained.
The DC Power Supply: The Driving Force
This is the external engine of the system, often a battery or a rectifier. It drives the non-spontaneous reaction by creating an electrical potential difference across the electrodes.
The power supply pumps electrons into the cathode, making it negative, and removes them from the anode, making it positive. Crucially, it must be a Direct Current (DC) source to maintain this fixed polarity.
How the System Works in Unison
The components are not independent; they form an integrated system where each part's function enables the next.
1. Establishing the Electrical Field
The process begins when the power supply is turned on. It immediately establishes a positive charge on the anode and a negative charge on the cathode.
2. Ion Migration
This electrical potential exerts a force on the ions within the electrolyte. Positively charged cations are drawn towards the negative cathode, while negatively charged anions are drawn towards the positive anode.
3. The Chemical Transformation
When the ions reach their respective electrodes, the electron exchange occurs. The substance is broken down as new compounds or elements are formed at the electrode surfaces, such as generating hydrogen gas at the cathode and oxygen gas at the anode during the electrolysis of water.
Common Pitfalls and Considerations
A functional understanding requires acknowledging the practical factors that influence the outcome and efficiency of the process.
Electrode Material Is Not Always Inert
While many systems use inert electrodes (like platinum or carbon) that only facilitate the reaction, some applications use active electrodes. These electrodes participate in the reaction, dissolving or being plated with metal, as seen in electroplating or refining.
The Separator Is Often Necessary
In many industrial applications, a separator or membrane is placed between the anode and cathode. This physical barrier allows ions to pass through but prevents the newly formed products from mixing and reacting with each other, which would decrease purity and efficiency.
Electrolyte Purity and Concentration Matter
The efficiency of the cell is directly tied to the electrolyte's ability to conduct ions. Impurities can cause unwanted side reactions, while incorrect concentration can impede ion flow and slow the entire process.
Applying This to Your Goal
Your design and operational focus will depend entirely on the desired outcome of the electrolysis.
- If your primary focus is high-purity products: Prioritize a high-quality separator membrane and a pure electrolyte to prevent cross-contamination and side reactions.
- If your primary focus is energy efficiency: Select highly conductive electrode materials and minimize the physical distance between them to reduce electrical resistance.
- If your primary focus is long-term stability: Use durable, inert electrode materials that resist corrosion from the electrolyte and the reaction products.
By understanding how these core components interact, you can manipulate chemical reactions with precision and control.
Summary Table:
| Component | Key Function |
|---|---|
| Electrodes (Anode & Cathode) | Sites of chemical change (Oxidation & Reduction) |
| Electrolyte | Conducts ions to complete the internal electrical circuit |
| DC Power Supply | Drives the non-spontaneous reaction by creating a potential difference |
| Separator/Membrane (Common) | Prevents product mixing, increasing purity and efficiency |
Ready to build or optimize your electrolysis system? KINTEK specializes in providing high-quality lab equipment and consumables for precise electrochemical processes. Whether your goal is high-purity product generation, energy efficiency, or long-term stability, our experts can help you select the right components. Contact our team today to discuss your laboratory's specific needs!
Visual Guide
Related Products
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What structural advantages do PEM electrolyzers offer? Compact, High-Density Hydrogen Production Solutions
- How do specialized electrolytic cells facilitate electrochemical testing? Enhance Stainless Steel Corrosion Analysis
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- What are the correct procedures to follow after using the electrolytic cell? Ensure Safety and Equipment Longevity
- What is a proton exchange membrane? The Selective Heart of Hydrogen Energy Systems



















