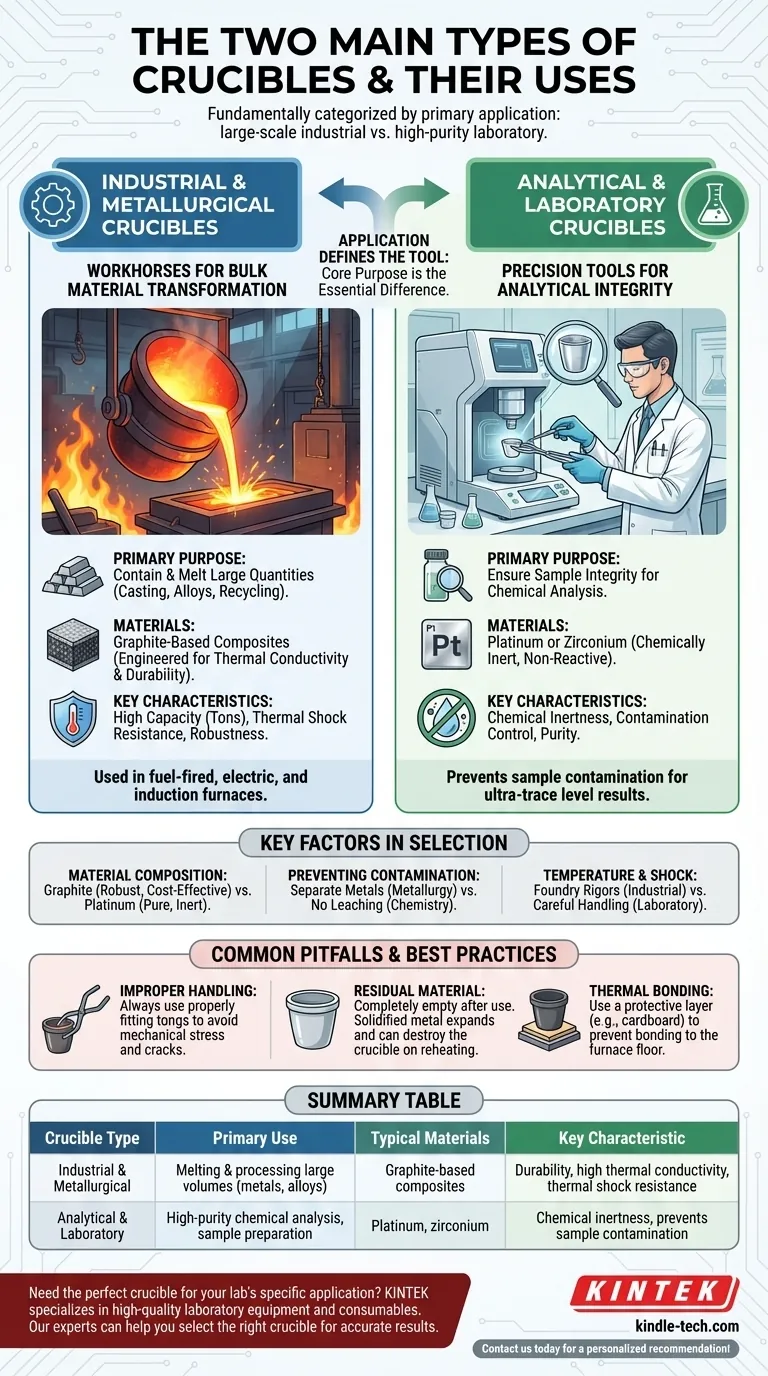
While crucibles have many variations, they are fundamentally categorized into two main types based on their primary application: large-scale industrial crucibles designed for melting and processing materials, and high-purity laboratory crucibles designed for precise chemical analysis and experimentation. The material, size, and design of a crucible are all dictated by which of these two functions it must serve.
The essential difference between crucible types is their core purpose. Industrial crucibles are built for bulk material transformation, prioritizing durability and capacity, while laboratory crucibles are built for analytical integrity, prioritizing chemical inertness to ensure sample purity.

The Core Distinction: Application Defines the Tool
A crucible is not a one-size-fits-all device. Its form is dictated entirely by its function, leading to a clear divergence between tools used for mass production and those used for precise measurement.
Type 1: Industrial & Metallurgical Crucibles
These are the workhorses of the foundry and materials science industries. Their primary purpose is to contain and melt large quantities of material, such as metals for casting, creating alloys, or recycling scrap.
Modern industrial crucibles are typically graphite-based composite materials. This composition is engineered for excellent thermal conductivity, resistance to thermal shock, and durability to withstand repeated heating and cooling cycles.
They are produced in a vast range of sizes, from small vessels to massive containers capable of holding several tons of molten metal, and are used in various fuel-fired, electric, and induction furnaces.
Type 2: Analytical & Laboratory Crucibles
In a laboratory setting, the goal shifts from processing volume to ensuring the integrity of a small sample. These crucibles are used for tasks like determining the precise chemical makeup of a substance or preparing samples for analysis.
The defining characteristic of these crucibles is their chemical inertness. They are made from materials like platinum or zirconium that will not react with or contaminate the sample, even at extreme temperatures.
Contamination control is paramount. Using such a highly inert crucible ensures that any results from an analysis reflect the sample itself, not impurities introduced by the container.
Key Factors in Crucible Selection
Understanding the two primary types of crucibles reveals the underlying principles that guide their design and selection.
Material Composition
The choice between a graphite composite and an inert metal like platinum is a direct reflection of the crucible's job. Graphite offers robust, cost-effective performance for melting, while platinum offers the purity required for ultra-trace level analysis.
Preventing Contamination
Both types of users want to avoid contamination, but the definition differs. In metallurgy, this means using separate crucibles for different metals to prevent unwanted alloying. In analytical chemistry, it means preventing any foreign element from the crucible itself from leaching into the analyte.
Temperature and Thermal Shock
All crucibles must withstand high temperatures, but their design also accounts for the stresses of use. Industrial crucibles are built to survive the rigors of a foundry, while laboratory crucibles, though robust, require careful handling to protect their expensive and specialized material.
Common Pitfalls and Best Practices
Regardless of the type, proper handling is essential for safety and performance. Failing to follow best practices can lead to equipment failure, contaminated results, or dangerous situations.
Improper Handling
Crucibles should always be handled with properly fitting tongs. This prevents mechanical stress that can cause cracks and damage, especially when the crucible is hot and more vulnerable.
Residual Material
A crucible must be completely emptied after each use. Metal left to solidify inside can expand upon reheating, exerting immense pressure that can easily crack and destroy the crucible.
Thermal Bonding
When placing a crucible in a furnace, it can be beneficial to place a protective layer, such as cardboard, between the furnace base and the crucible. This prevents the crucible from bonding to the furnace floor at high temperatures, which can damage it upon removal.
Making the Right Choice for Your Goal
Your objective determines your tool. To select the correct crucible, you must first be clear about the intended outcome of your high-temperature work.
- If your primary focus is melting, casting, or creating alloys: You need an industrial, graphite-based composite crucible designed for durability and thermal performance.
- If your primary focus is high-purity chemical analysis or sample preparation: You must use a laboratory crucible made from an inert material like platinum or zirconium to guarantee sample integrity.
Ultimately, understanding this fundamental division is the key to achieving successful and accurate results in any high-temperature application.
Summary Table:
| Crucible Type | Primary Use | Typical Materials | Key Characteristic |
|---|---|---|---|
| Industrial & Metallurgical | Melting & processing large volumes of materials (e.g., metals, alloys) | Graphite-based composites | Durability, high thermal conductivity, thermal shock resistance |
| Analytical & Laboratory | High-purity chemical analysis, sample preparation | Platinum, zirconium | Chemical inertness, prevents sample contamination |
Need the perfect crucible for your lab's specific application? KINTEK specializes in high-quality laboratory equipment and consumables, including a full range of industrial and analytical crucibles. Our experts can help you select the right crucible to ensure accurate results, prevent contamination, and maximize efficiency. Contact us today to discuss your needs and get a personalized recommendation!
Visual Guide
Related Products
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- High Purity Pure Graphite Crucible for Electron Beam Evaporation
People Also Ask
- Why are alumina crucibles preferred for DSC analysis of SiCp/2009Al? Ensure High-Temperature Chemical Inertness
- How does a ceramic crucible with a lid function within a muffle furnace? Ensure Precise Biomass Volatile Matter Analysis
- What is a porcelain crucible? Your Essential Guide to High-Temp Lab Work
- Why is a graphite crucible with a plug used for Mg3Sb2 alloys? Ensure Stoichiometric Precision in P-Type Synthesis
- What are the advantages of using a Glassy Carbon Crucible for fluoride salts? Ensure Purity up to 1000°C
- What size crucible do I need? A Guide to Safe and Efficient Material Melting
- What are the parts of a crucible furnace? A Guide to Its Core Components and Function
- What can I use as a crucible for melting gold? Choose the Right Material for a Clean, Efficient Melt



















