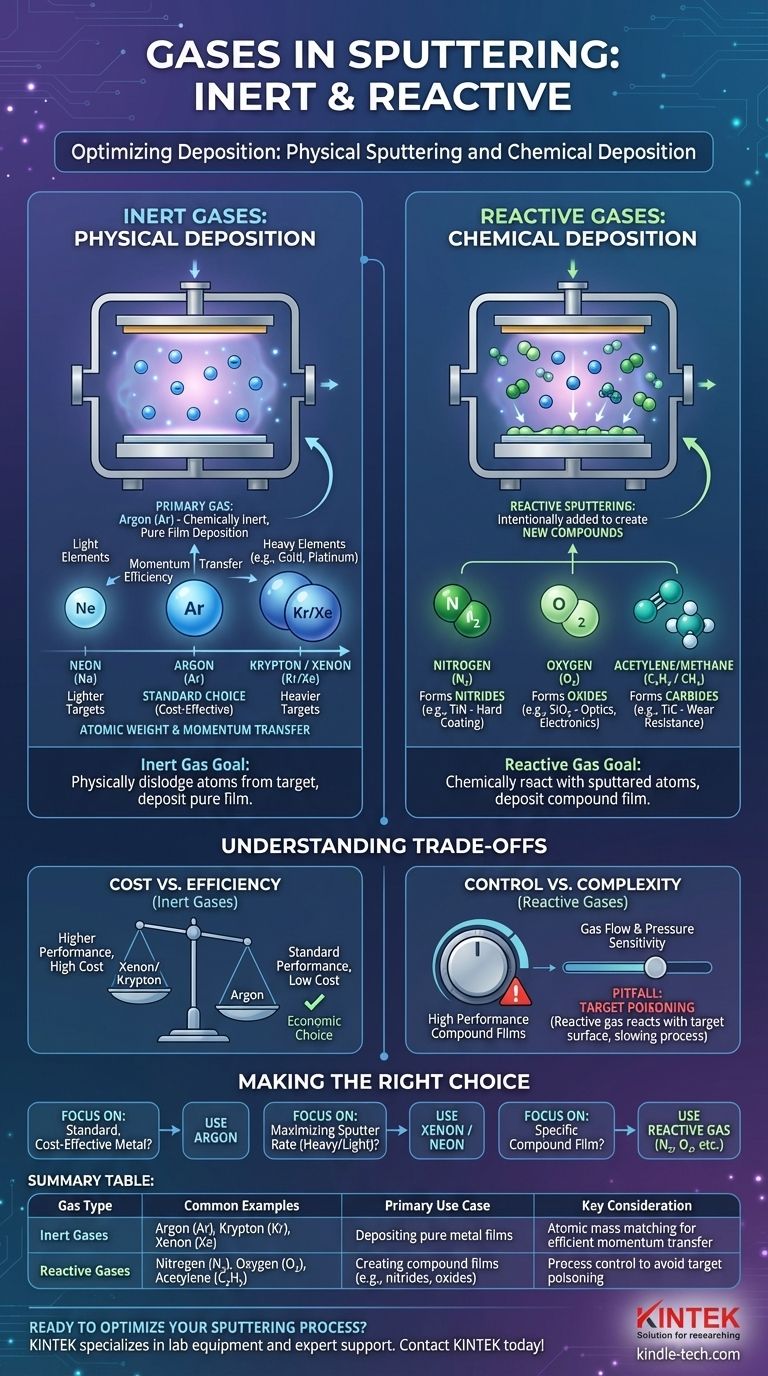
In sputtering, the primary and most common gas used is argon. However, the complete answer includes other noble gases like neon, krypton, and xenon for physical sputtering, as well as reactive gases like nitrogen and oxygen, which are intentionally used to create new chemical compounds during the deposition process.
The choice of gas in sputtering is a critical process parameter. You either select an inert gas for purely physical deposition based on atomic weight and efficiency, or you introduce a reactive gas to intentionally form a new chemical compound on your substrate.

The Role of Inert Gases: Physical Deposition
In its most common form, sputtering is a physical process. The goal is to physically dislodge atoms from a source material (the target) and deposit them onto a substrate, much like a microscopic sandblaster.
The Fundamental Mechanism
The process begins by introducing a low-pressure gas, typically argon, into a vacuum chamber. An electric field is applied, which strips electrons from the gas atoms, creating a glowing, ionized gas known as plasma.
These positively charged gas ions are then accelerated into the negatively charged target material. The resulting high-energy collisions have enough force to knock atoms loose from the target, which then travel and deposit as a thin film on the substrate.
Why Argon is the Default Choice
Argon is the workhorse of the sputtering industry for a few key reasons. As a noble gas, it is chemically inert, meaning it will not react with the target material.
This ensures that a pure film of the target material is deposited. It also offers a good balance of atomic mass and cost-effectiveness for a wide range of common target materials.
The Principle of Momentum Transfer
For the most efficient sputtering process, the atomic weight of the gas ion should be close to the atomic weight of the target atom. This is like trying to knock a bowling ball over; another bowling ball is far more effective than a tennis ball.
Because of this principle, argon isn't always the optimal choice.
Choosing Other Inert Gases
Neon (Ne), being lighter than argon, is more effective for sputtering very light target elements.
Krypton (Kr) and Xenon (Xe) are much heavier than argon. They provide significantly more efficient momentum transfer and higher deposition rates when sputtering heavy elements like gold or platinum.
The Role of Reactive Gases: Chemical Deposition
Sometimes, the goal isn't to deposit a pure material but to create a specific chemical compound, such as a ceramic or an oxide. This is achieved through reactive sputtering.
Defining Reactive Sputtering
In this process, a reactive gas is intentionally added to the inert sputtering gas (like argon). This gas reacts with the sputtered atoms as they travel from the target to the substrate.
The result is the deposition of a compound film that has entirely different properties—such as hardness, color, or electrical resistance—from the original target material.
Common Reactive Gases and Their Products
The choice of reactive gas directly determines the final compound.
- Nitrogen (N₂) is used to form nitride films, such as Titanium Nitride (TiN), a common hard coating.
- Oxygen (O₂) is used to form oxide films, such as Silicon Dioxide (SiO₂), a crucial material in optics and electronics.
- Acetylene (C₂H₂) or Methane (CH₄) can be used to form carbide films like Titanium Carbide (TiC).
Understanding the Trade-offs
Choosing a sputtering gas involves balancing performance, cost, and process complexity.
Inert Gas Selection: Cost vs. Efficiency
While xenon might provide the highest sputtering rate for a heavy target, it is also significantly more expensive than argon. For most applications, the performance boost from xenon does not justify the added operational cost, making argon the default economic choice.
Reactive Sputtering: Control vs. Complexity
Reactive sputtering enables the creation of high-performance compound films that would be difficult or impossible to make otherwise. However, the process is far more complex to control. The final film's chemical composition (stoichiometry) is extremely sensitive to gas flow rates and pressures.
The Pitfall of Target Poisoning
A common problem in reactive sputtering is target poisoning. This occurs when the reactive gas reacts with the surface of the target itself, forming a compound layer (e.g., an oxide layer). This new layer often has a much lower sputter rate, which can slow or even halt the deposition process.
Making the Right Choice for Your Goal
Your choice of gas should be dictated entirely by the properties you need in your final thin film.
- If your primary focus is standard, cost-effective metal deposition: Use argon. It is the reliable and economical industry standard for sputtering pure metals like gold, copper, or aluminum.
- If your primary focus is maximizing the sputter rate of a very heavy or light element: Consider using xenon (for heavy targets) or neon (for light targets) to achieve more efficient momentum transfer.
- If your primary focus is depositing a specific compound film (e.g., a hard, optical, or dielectric coating): Employ reactive sputtering by mixing nitrogen, oxygen, or another reactive gas with your primary argon flow.
Ultimately, selecting the right gas transforms sputtering from a simple coating technique into a precise materials engineering tool.
Summary Table:
| Gas Type | Common Examples | Primary Use Case | Key Consideration |
|---|---|---|---|
| Inert Gases | Argon (Ar), Krypton (Kr), Xenon (Xe) | Depositing pure metal films | Atomic mass matching for efficient momentum transfer |
| Reactive Gases | Nitrogen (N₂), Oxygen (O₂), Acetylene (C₂H₂) | Creating compound films (e.g., nitrides, oxides) | Process control to avoid target poisoning |
Ready to Optimize Your Sputtering Process?
The right gas choice is critical for achieving the desired film properties, whether you need a pure metal coating or a specific compound like a nitride or oxide. KINTEK specializes in providing the lab equipment, consumables, and expert support to help you master your deposition process. Let our team help you select the ideal gases and configuration for your application.
Contact KINTEK today to discuss your sputtering needs and enhance your lab's capabilities!
Visual Guide
Related Products
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
People Also Ask
- What are the examples of chemical deposition? From CVD to Plating, Find Your Coating Method
- What is the CVD method of chemical vapour deposition? A Guide to High-Performance Thin Films
- What is a thin film coating? Engineer New Surface Properties for Your Substrate
- What are the components of the CVD system? A Guide to the Core Modules for Thin Film Deposition
- What is a major challenge in synthesizing bulk materials using gas-to-particle CVD? Solve the Aggregation Hurdle
- What is chemical deposition method? A Guide to High-Performance Thin Film Fabrication
- Why is a rotating sample holder used for stainless steel deposition? Achieving Maximum Coating Uniformity
- What is sputtering in metal deposition techniques? Achieve Superior Thin-Film Coatings














