In furnace heat treatment, a steam atmosphere is used for two primary purposes: It provides a method for the scale-free tempering and stress-relieving of ferrous metals, and it enhances the physical properties of sintered iron parts. For these processes to be effective, the metal surfaces must be thoroughly cleaned and free of any existing oxides before treatment within the required temperature range of 345°C to 650°C (655°F to 1200°F).
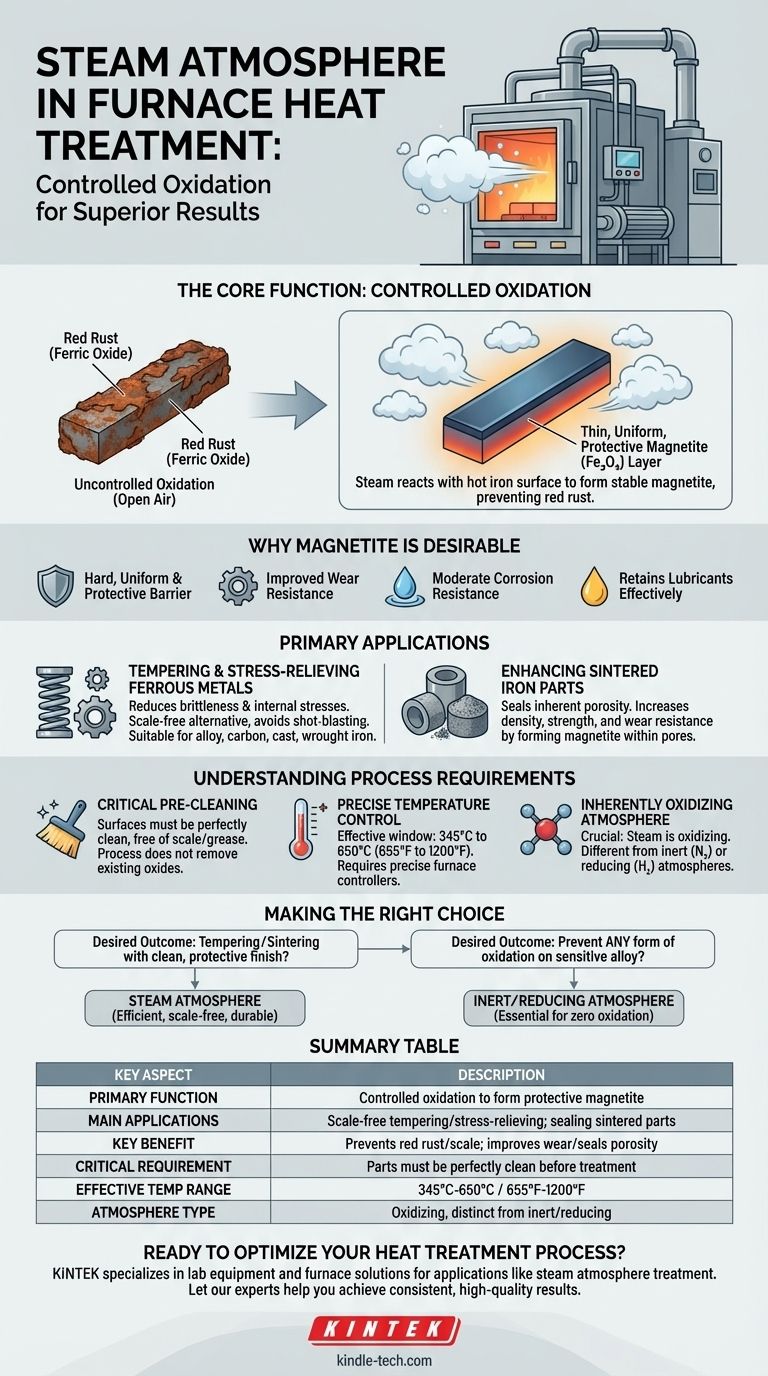
The core function of a steam atmosphere is not to prevent all oxidation, but to control it. It intentionally creates a thin, uniform, and protective layer of blue-black magnetite (iron oxide) that prevents the formation of destructive, flaky red rust or scale.

The Core Function: Controlled Oxidation
What "Scale-Free" Really Means
The term "scale-free" can be misleading. It does not mean a complete absence of an oxide layer.
Instead, it refers to the prevention of the thick, flaky, and undesirable red rust (ferric oxide) that typically forms when heating iron-based metals in open air.
The Formation of Magnetite (Fe₃O₄)
When superheated steam is introduced into the furnace, it reacts with the hot iron surface.
This reaction forms a thin, tightly adhering, and stable layer of blue-black iron oxide known as magnetite. This controlled layer passivates the surface.
Why Magnetite is Desirable
Unlike loose scale, the magnetite layer is hard, uniform, and protective. It acts as a barrier, preventing further, deeper oxidation of the component.
This finish also improves wear resistance, provides moderate corrosion resistance, and creates a surface that can retain lubricants effectively.
Primary Applications of Steam Treatment
Tempering and Stress-Relieving Ferrous Metals
This is the most common application. After hardening, steel parts are often tempered to reduce brittleness and relieve internal stresses.
Using a steam atmosphere allows this to be done without the costly and time-consuming cleaning steps (like shot-blasting) required to remove scale formed during open-air heating. It's suitable for alloy, carbon, cast, and wrought iron.
Enhancing Sintered Iron Parts
Sintered parts, made from powdered metal, are inherently porous.
Steam treatment is exceptionally effective here because the magnetite forms within the pores, effectively sealing the surface. This process significantly reduces porosity and increases the part's density, strength, and wear resistance.
Understanding the Process Requirements
Critical Pre-Cleaning
The success of steam treatment is entirely dependent on the initial state of the part.
The surfaces must be perfectly clean and free of any pre-existing scale, grease, or rust. The steam process creates a new oxide layer; it does not remove an existing one.
Precise Temperature and Atmosphere Control
The process is only effective within a specific temperature window of 345°C to 650°C.
Modern atmosphere furnaces use precise controllers to maintain both the temperature and the composition of the steam atmosphere, ensuring consistent and reliable results.
An Inherently Oxidizing Environment
It is crucial to recognize that steam is an oxidizing atmosphere.
While it's used to prevent unwanted scaling, it is fundamentally different from inert (e.g., nitrogen) or reducing (e.g., hydrogen) atmospheres, which are used when the goal is to prevent any form of oxidation on the metal's surface.
Making the Right Choice for Your Goal
Selecting the correct furnace atmosphere depends entirely on the desired outcome for your material.
- If your primary focus is tempering or stress-relieving iron parts with a clean, protective finish: Steam treatment offers an efficient, scale-free alternative to open-air heating.
- If your primary focus is improving the density and wear resistance of sintered iron components: Steam is an excellent choice for sealing porosity and creating a durable surface.
- If your primary focus is preventing any form of oxidation on a sensitive alloy: You must use an inert or reducing atmosphere, as the steam process is inherently oxidizing.
Ultimately, using a steam atmosphere is a strategic choice to create a specific, beneficial oxide layer for a targeted set of applications.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Function | Controlled oxidation to form a protective magnetite (Fe₃O₄) layer. |
| Main Applications | Scale-free tempering/stress-relieving of ferrous metals; sealing & strengthening sintered iron parts. |
| Key Benefit | Prevents destructive red rust/scale; improves wear resistance and seals porosity. |
| Critical Requirement | Parts must be thoroughly cleaned and free of existing oxides before treatment. |
| Effective Temperature Range | 345°C to 650°C (655°F to 1200°F). |
| Atmosphere Type | Oxidizing (fundamentally different from inert or reducing atmospheres). |
Ready to Optimize Your Heat Treatment Process?
Choosing the right furnace atmosphere is critical for achieving your desired material properties. KINTEK specializes in lab equipment and consumables, providing the precise furnace solutions you need for applications like steam atmosphere treatment.
Let our experts help you:
- Select the ideal furnace for your specific tempering or sintering requirements.
- Achieve consistent, high-quality results with reliable temperature and atmosphere control.
- Enhance your lab's efficiency and improve the durability of your metal components.
Contact us today to discuss how our solutions can bring value to your laboratory. Get in Touch via Our Contact Form
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
People Also Ask
- What is an inert condition? A Guide to Preventing Fires and Explosions
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab
- What gases are used in inert atmospheres? Choose the Right Gas for Non-Reactive Environments
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety



















