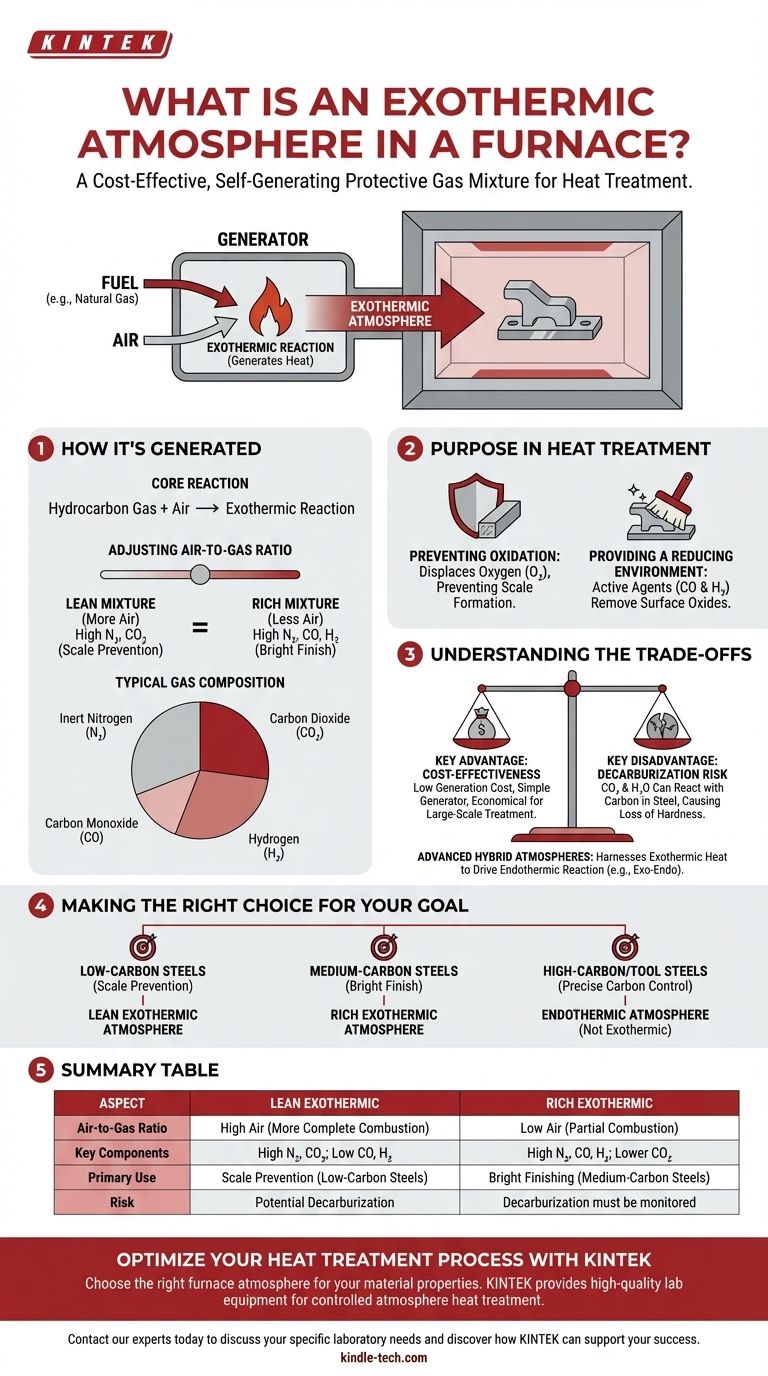
At its core, an exothermic atmosphere is a protective gas mixture used in heat-treating furnaces that is created by a chemical reaction that generates its own heat. This process involves the controlled combustion of a hydrocarbon fuel (like natural gas) with air, creating an environment that actively prevents the metal's surface from oxidizing or forming scale during treatment.
The critical concept is that an exothermic atmosphere is a cost-effective way to displace oxygen and protect metals, but its composition offers less precise control compared to more complex and expensive furnace atmospheres.

How an Exothermic Atmosphere is Generated
The name "exothermic" directly refers to the generation process, where a chemical reaction releases energy in the form of heat. This self-sustaining reaction is the defining characteristic.
The Core Reaction
The atmosphere is created in a dedicated generator by burning a precise mixture of hydrocarbon gas and air. This partial combustion is an exothermic reaction, meaning it does not require a continuous external heat source to proceed once initiated.
Adjusting the Air-to-Gas Ratio
The properties of the final atmosphere are controlled by the ratio of air to gas fed into the generator.
- A "lean" mixture uses more air, resulting in more complete combustion. The resulting gas is high in nitrogen and carbon dioxide with low levels of reducing agents.
- A "rich" mixture uses less air, resulting in partial combustion. This produces a gas higher in carbon monoxide (CO) and hydrogen (H₂), which are powerful reducing agents.
Typical Gas Composition
After the reaction and cooling to remove excess water vapor, the resulting atmosphere is primarily composed of inert nitrogen (N₂) from the air. The balance consists of carbon dioxide (CO₂), carbon monoxide (CO), and hydrogen (H₂), with the exact percentages determined by the richness of the initial mixture.
The Purpose in Heat Treatment
Using a controlled atmosphere is fundamental to achieving specific metallurgical properties and surface finishes. An exothermic atmosphere serves two primary functions.
Preventing Oxidation
The most basic function is to displace oxygen from the furnace chamber. By filling the furnace with the generated gas, there is no free oxygen available to react with the hot metal surface, which prevents the formation of undesirable oxides and scale.
Providing a Reducing Environment
A rich exothermic atmosphere contains active reducing agents, specifically **carbon monoxide (CO) and hydrogen (H₂) **. These gases can chemically react with and remove any light oxides that may already be on the metal's surface, resulting in a cleaner, brighter finish after treatment.
Understanding the Trade-offs
While effective, an exothermic atmosphere is not suitable for every application. Understanding its limitations is key to using it correctly.
Key Advantage: Cost-Effectiveness
The primary benefit of an exothermic atmosphere is its low generation cost. It uses relatively inexpensive natural gas and air in a simple generator, making it a highly economical choice for large-scale, general-purpose heat treating.
Key Disadvantage: Decarburization Risk
The presence of carbon dioxide (CO₂) and water vapor (H₂O), especially in lean mixtures, can be detrimental to high-carbon steels. These compounds can react with carbon in the steel's surface, leading to a loss of hardness—a defect known as decarburization.
Advanced Hybrid Atmospheres
In some specialized processes, the heat from an exothermic reaction is harnessed to drive a secondary, endothermic reaction. This creates a hybrid "exo-endo" atmosphere with a tailored composition, such as reduced hydrogen content to minimize the risk of hydrogen embrittlement in sensitive parts.
Making the Right Choice for Your Goal
Selecting the correct furnace atmosphere is critical for achieving the desired metallurgical outcome without causing unintended surface defects.
- If your primary focus is cost-effective scale prevention on low-carbon steels: A lean exothermic atmosphere is an excellent and economical choice for processes like annealing or normalizing.
- If your primary focus is bright finishing of medium-carbon steels: A rich exothermic atmosphere provides better reducing potential to keep surfaces clean, but decarburization must be monitored.
- If your primary focus is carburizing or treating high-carbon or tool steels: A more controllable and potent endothermic atmosphere is necessary to prevent decarburization and precisely manage surface carbon content.
Understanding the chemistry and trade-offs of each atmosphere allows you to protect your materials while optimizing your process for efficiency and cost.
Summary Table:
| Aspect | Lean Exothermic | Rich Exothermic |
|---|---|---|
| Air-to-Gas Ratio | High Air (More Complete Combustion) | Low Air (Partial Combustion) |
| Key Components | High N₂, CO₂; Low CO, H₂ | High N₂, CO, H₂; Lower CO₂ |
| Primary Use | Scale Prevention (Low-Carbon Steels) | Bright Finishing (Medium-Carbon Steels) |
| Risk | Potential Decarburization | Decarburization must be monitored |
Optimize Your Heat Treatment Process with KINTEK
Choosing the right furnace atmosphere is critical for achieving the desired material properties and surface finish. Whether you are annealing low-carbon steels or require a bright finish on medium-carbon steels, the correct atmosphere is key to preventing defects like oxidation and decarburization.
KINTEK specializes in providing high-quality lab equipment and consumables, including solutions for controlled atmosphere heat treatment. Our expertise can help you select the right equipment and processes to improve efficiency, reduce costs, and ensure consistent, high-quality results.
Contact our experts today to discuss your specific laboratory needs and discover how KINTEK can support your success.
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- Can nitrogen be used for brazing? Key Conditions and Applications Explained
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab
- What is the purpose of using an atmosphere-controlled heating furnace for Cu reduction? Achieve Active Catalytic States
- How do you make an inert atmosphere? Master Safe, Pure Processes with Inerting



















