At its core, freeze drying is a sophisticated dehydration technique. Also known as lyophilization, the process works by first completely freezing a material, then placing it under a deep vacuum. This combination of cold and low pressure allows the frozen water to turn directly from a solid (ice) into a gas (vapor) without ever passing through a damaging liquid phase.
The central principle of freeze drying is not just to remove water, but to do so while preserving a material's original structure and integrity. By turning ice directly into vapor through sublimation, it bypasses the destructive forces of conventional drying, making it the gold standard for sensitive products.

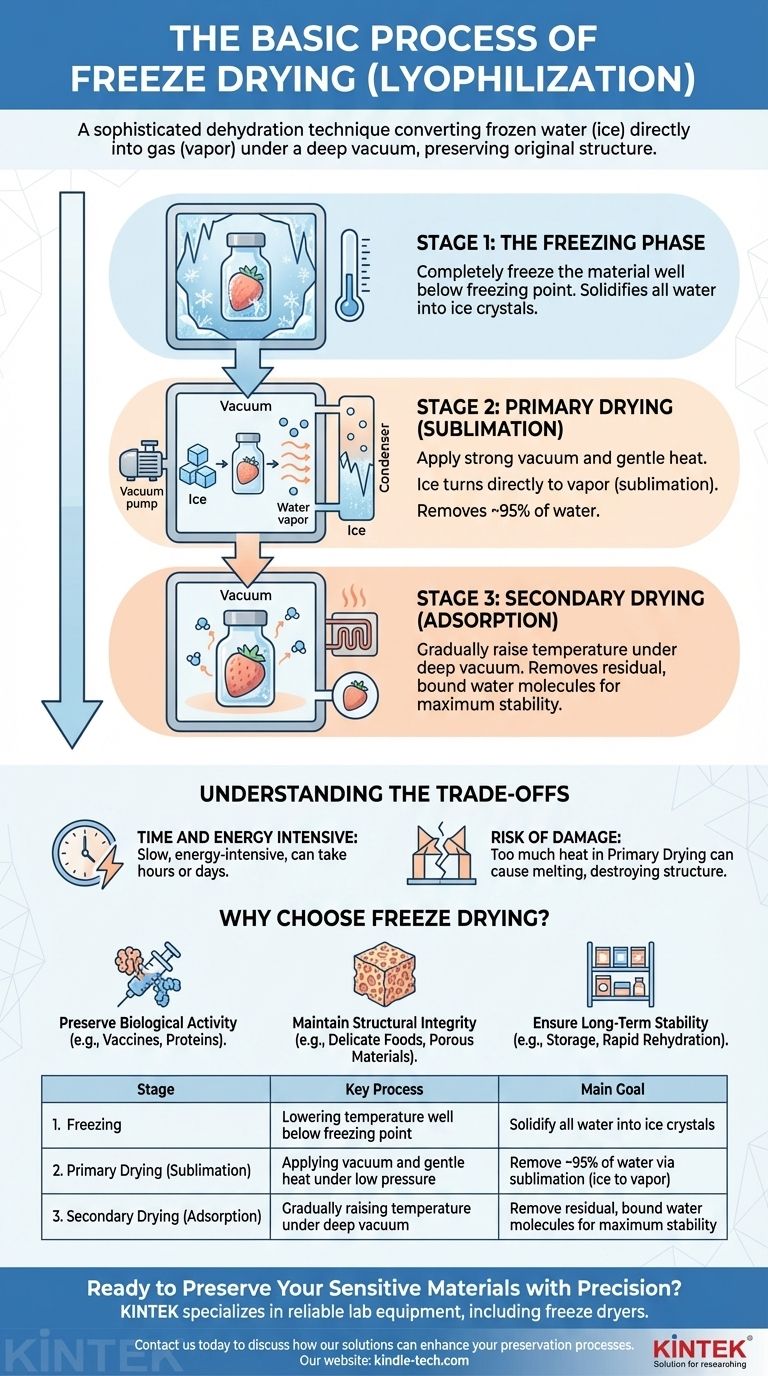
The Three Critical Stages of Lyophilization
The entire freeze-drying process is a carefully controlled, multi-stage operation. Each phase serves a distinct purpose in removing water while protecting the product's fundamental characteristics.
Stage 1: The Freezing Phase
The first and most essential step is to completely freeze the material. The product's temperature is lowered well below its freezing point to ensure all water is converted into a solid state.
How a product is frozen directly impacts the size of the ice crystals formed, which can influence the speed of the subsequent drying phase and the quality of the final product.
Stage 2: Primary Drying (Sublimation)
This is the main event of the process. Once frozen, the material is placed under a strong vacuum, significantly reducing the surrounding pressure.
A small amount of heat is then carefully introduced. This energy gives the ice molecules just enough energy to break free and transform directly into water vapor, a process called sublimation.
This vapor is then drawn away and collected on a very cold surface called a condenser, which turns it back into ice, trapping it away from the product. This stage removes the vast majority of the water, typically around 95%.
Stage 3: Secondary Drying (Adsorption)
After primary drying, a small amount of water molecules remain tightly bound to the material. The goal of this final stage is to remove this residual moisture.
To accomplish this, the temperature is gradually raised and an even deeper vacuum may be applied. This gives the bound water molecules the energy needed to detach, ensuring the product is as dry and stable as possible.
Understanding the Trade-offs
While highly effective, freeze drying is a deliberate choice with specific implications. It is not the right solution for every dehydration need.
The Cost of Precision: Time and Energy
Lyophilization is a slow and energy-intensive process. The careful, multi-stage approach can take a significant amount of time, often lasting for many hours or even days depending on the material.
This makes it considerably more expensive than conventional drying methods like heat or air drying.
The Risk of Structural Damage
The primary benefit of freeze drying is its gentle nature, but it is not foolproof. Applying too much heat during the primary drying stage can cause the ice to melt instead of sublimate.
This can alter or destroy the delicate structure you are trying to preserve, defeating the entire purpose of using the process. Proper calibration and control are paramount to success.
Why Choose Freeze Drying?
The decision to use freeze drying should be based on the specific preservation requirements of your material.
- If your primary focus is preserving biological activity: For sensitive materials like vaccines, proteins, enzymes, or antibodies, lyophilization is often the only viable method to maintain their effectiveness.
- If your primary focus is maintaining structural integrity: For delicate foods or porous materials where texture, shape, and appearance are critical, this process provides unparalleled results.
- If your primary focus is long-term stability: Freeze drying creates a final product that is lightweight, porous, and highly stable, making it ideal for storage and rapid rehydration.
Ultimately, freeze drying is the definitive choice when the goal is preservation without compromise.
Summary Table:
| Stage | Key Process | Main Goal |
|---|---|---|
| 1. Freezing | Lowering temperature well below freezing point | Solidify all water into ice crystals |
| 2. Primary Drying (Sublimation) | Applying vacuum and gentle heat under low pressure | Remove ~95% of water via sublimation (ice to vapor) |
| 3. Secondary Drying (Adsorption) | Gradually raising temperature under deep vacuum | Remove residual, bound water molecules for maximum stability |
Ready to Preserve Your Sensitive Materials with Precision?
Freeze drying is essential for maintaining the biological activity and structural integrity of sensitive products like pharmaceuticals, proteins, and delicate foods. KINTEK specializes in providing reliable lab equipment, including freeze dryers, to meet your specific laboratory needs.
Contact us today to discuss how our solutions can enhance your preservation processes and ensure long-term stability for your valuable materials.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Liquid Nitrogen Cryogenic Grinder Mill Cryomill Airflow Ultrafine Pulverizer
- Touchscreen Automatic Vacuum Heat Press
People Also Ask
- What industries can benefit from freeze drying technology? Preserve Value in Pharma, Food & More
- What is the rehydration capability of freeze-dried products? Achieve Superior Quality & Instant Restoration
- Where are evaporators used in food industry? Concentrate Products & Reduce Costs
- How do you evaporate DMSO solvent? Master Gentle, High-Vacuum Techniques for Sensitive Samples
- What physical property enhancements does freeze drying provide for pharmaceutical products? Achieve Superior Stability & Global Distribution



















