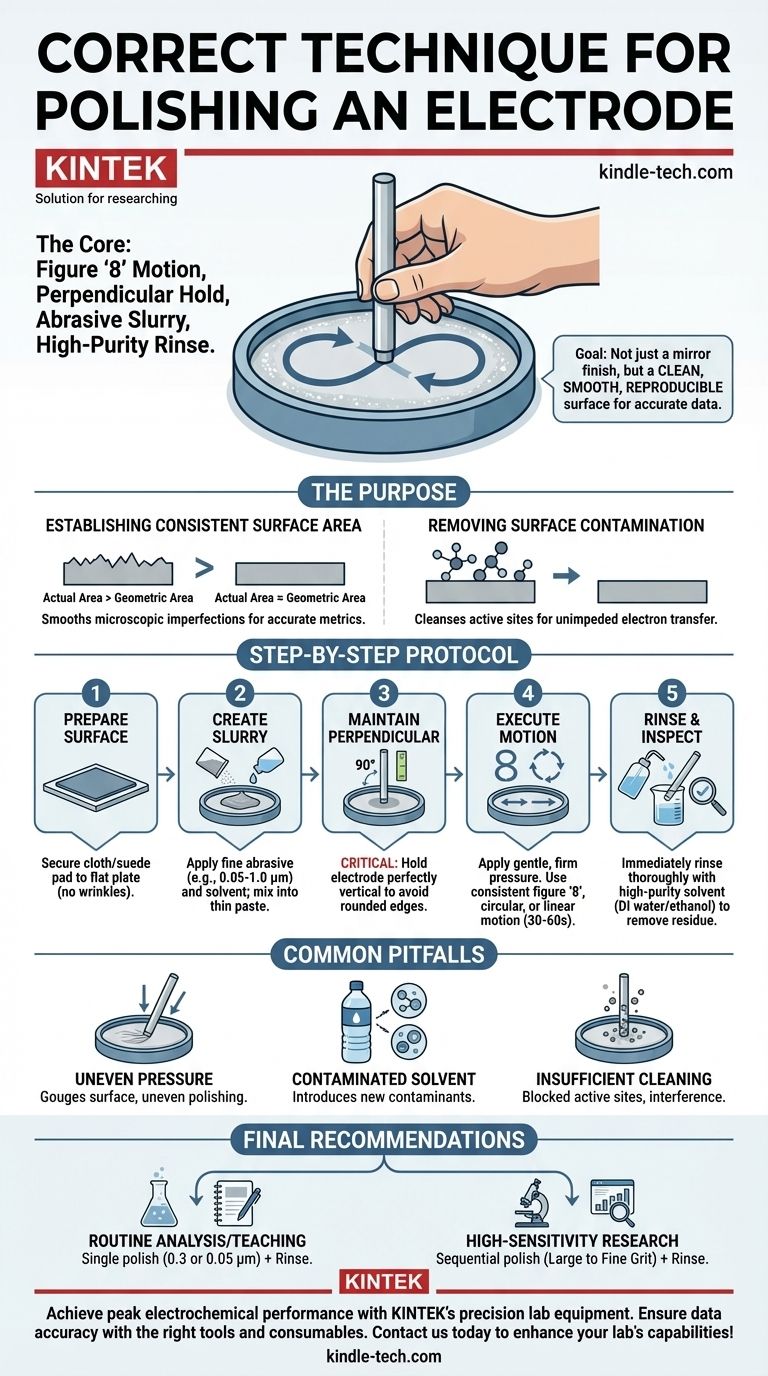
The correct technique for polishing an electrode involves moving it in a consistent pattern, such as a figure '8', across a polishing pad wetted with a fine abrasive slurry. Crucially, the electrode must be held perfectly perpendicular to the pad's surface to ensure an even finish. The process is completed by thoroughly rinsing the electrode with a high-purity solvent like deionized water or ethanol to remove all residues.
The goal of electrode polishing is not simply to create a mirror finish, but to produce a clean, smooth, and electrochemically reproducible surface. Proper technique is the foundation for obtaining accurate and reliable experimental data.

The Core Purpose of Polishing
Before detailing the procedure, it is vital to understand why polishing is a non-negotiable step in most electrochemical experiments. The reasons are twofold and directly impact the quality of your results.
Establishing a Consistent Surface Area
Polishing smooths out microscopic scratches, pits, and grooves on the electrode's surface. This ensures the geometric area of the electrode face is as close as possible to its true, electrochemically active surface area.
An unpolished or poorly polished electrode has a rough, uneven surface, which increases its actual surface area. This discrepancy can lead to significant errors in calculated metrics like current density.
Removing Surface Contamination
The electrode surface is the site of electron transfer. Any unwanted species—such as adsorbed molecules from previous experiments, solvent residue, or oxides—can interfere with or inhibit the reaction you intend to study.
Polishing is a mechanical cleaning method that physically removes this layer of contamination, exposing a fresh, pristine surface for your experiment.
The Step-by-Step Polishing Protocol
Achieving a properly polished electrode requires methodical execution. While specific materials may vary, the core principles remain constant.
1. Prepare the Polishing Surface
First, secure a polishing cloth or suede pad to a flat, stable plate, often made of glass. Ensure there are no wrinkles or debris trapped underneath the pad.
2. Create the Abrasive Slurry
Apply a small amount of a polishing powder, such as alumina, to the pad. The grit size is chosen based on the electrode's condition; a common sequence is to start with a larger grit (e.g., 1.0 µm) and finish with a very fine one (e.g., 0.05 µm).
Add a few drops of deionized water or ethanol and mix it into a thin, consistent paste or slurry.
3. Maintain Perpendicular Alignment
This is the most critical mechanical aspect of the technique. Hold the electrode so its body is perfectly perpendicular (90 degrees) to the polishing pad.
Any deviation from this vertical alignment will round the edges of the electrode, altering its defined geometric area and leading to inaccurate data.
4. Execute the Polishing Motion
With gentle but firm pressure, move the electrode across the slurry-coated pad. Three motions are standard and effective:
- A continuous figure '8' pattern.
- A circular pattern (clockwise or counter-clockwise).
- A linear, back-and-forth motion.
The key is to use a consistent motion to ensure the entire surface is abraded evenly. Polish for approximately 30-60 seconds.
5. Rinse and Inspect
After polishing, immediately and thoroughly rinse the electrode surface with deionized water or ethanol. Use a squirt bottle to dislodge any remaining abrasive particles.
You may sonicate the electrode in the rinsing solvent for a minute to ensure it is completely clean, but be mindful of the electrode's construction materials.
Common Pitfalls and How to Avoid Them
Even with the right steps, small errors can compromise your results. Being aware of these common mistakes is crucial for developing a reliable technique.
Uneven Pressure
Applying too much or inconsistent pressure can gouge the electrode surface or cause uneven polishing. The goal is a light, steady pressure that allows the abrasive slurry to do the work.
Contaminated Rinsing Solvent
Using tap water or a low-purity solvent to rinse the electrode can introduce new contaminants (like ions) onto the very surface you just cleaned. Always use high-purity, deionized water or an appropriate solvent like ethanol.
Insufficient Cleaning
Failing to rinse away all the polishing slurry is a common mistake. Any residual alumina particles left on the electrode surface will block active sites and interfere with your electrochemical measurement.
Final Recommendations for Your Experiment
Your approach to polishing should align directly with the sensitivity and goals of your work.
- If your primary focus is routine analysis or teaching: A single polishing step with 0.3 or 0.05 µm alumina followed by a thorough rinse is often sufficient for reliable results.
- If your primary focus is high-sensitivity research or surface studies: A sequential polishing procedure, moving from a larger grit (1.0 µm) down to the finest (0.05 µm), is necessary to create a pristine, ultra-smooth surface.
Ultimately, consistent and meticulous polishing is an investment that pays dividends in the form of clear, reproducible, and trustworthy data.
Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1. Prepare | Secure polishing pad | Ensure a stable, flat surface |
| 2. Slurry | Apply fine abrasive (e.g., alumina) | Create a consistent polishing paste |
| 3. Polish | Move in figure-8 pattern, hold perpendicular | Achieve an even, flat surface |
| 4. Rinse | Use high-purity solvent (DI water/ethanol) | Remove all abrasive residues |
| 5. Inspect | Check for a smooth, clean finish | Confirm a reproducible surface area |
Achieve peak electrochemical performance with KINTEK's precision lab equipment. Proper electrode polishing is fundamental, but it starts with the right tools. KINTEK specializes in high-quality lab supplies, including polishing materials and consumables, to support your research and ensure data accuracy. Let our experts help you select the ideal products for your specific application. Contact us today to enhance your lab's capabilities and reliability!
Visual Guide
Related Products
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Laboratory Grinding Mill Mortar Grinder for Sample Preparation
- Laboratory Hybrid Tissue Grinding Mill
- Platinum Auxiliary Electrode for Laboratory Use
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
People Also Ask
- What is the expected lifespan of a copper sulfate reference electrode? Maximize Longevity with Proper Maintenance
- Why is molybdenum-containing stainless steel selected as the cathode for fishmeal wastewater? Durable & Stable Design
- What are the available types of copper sulfate reference electrodes? Wood vs. Ceramic Core Explained
- What are the functions of the Ag/AgCl reference electrode and the platinum wire in (U1−xThx)O2 film oxidation studies?
- Why is a platinum wire counter electrode necessary for EIS on 8620 steel? Ensure Pure Data Accuracy
- What is the recommended pre-treatment procedure for gold or platinum sheets before use? Ensure a Pristine, Reproducible Surface
- What are the correct procedures for handling a titanium electrode after use? Extend Coating Life and Performance
- Why Use a Three-Electrode System for LPR Testing? Achieve Precision in Corrosion Measurement



















