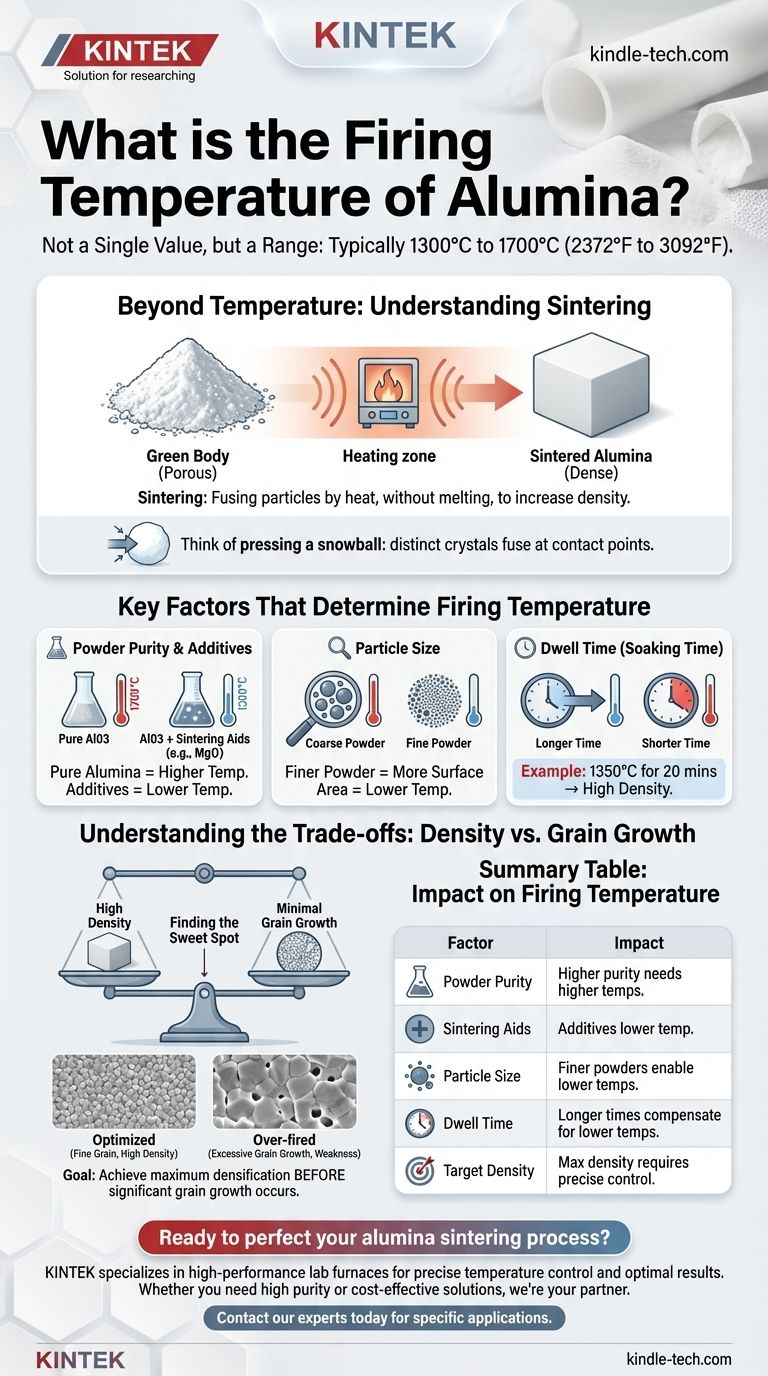
The firing temperature of alumina is not a single value, but rather a range typically between 1300°C and 1700°C (2372°F and 3092°F). The precise temperature depends entirely on the desired outcome, the purity of the alumina, and the processing time. For example, high-purity alumina powder can be fired to 99% of its theoretical density at a relatively low 1350°C, but only when held for a specific duration.
The central challenge is not finding one correct temperature, but understanding how temperature, time, and material purity interact. Mastering these variables is the key to controlling the final properties of your alumina component.

Beyond Temperature: Understanding the Sintering Process
To effectively use alumina, you must look beyond a simple temperature value and understand the underlying manufacturing process: sintering.
What is Sintering?
Sintering is the process of compacting and forming a solid mass of material by heat, but without melting it to the point of liquefaction.
Imagine pressing a snowball. The individual snow crystals are distinct. Sintering is the thermal process that fuses those individual crystals at their contact points, turning the loose collection of particles into a single, solid object.
The Goal: Densification
The primary goal of firing alumina is typically densification. The initial "green" body, formed from powder, is filled with tiny pores or voids between the particles.
Applying heat gives the atoms mobility, allowing them to diffuse across particle boundaries. This process closes the pores, shrinks the component, and dramatically increases its density. Higher density almost always correlates with greater strength, hardness, and impermeability.
Key Factors That Determine Firing Temperature
The 1350°C value from the reference is a useful data point, but it's only valid for a specific set of conditions. Changing any of the following variables will change the required temperature.
Powder Purity and Additives
Pure alumina has a very high melting point and requires significant thermal energy to sinter effectively. This often means higher temperatures are necessary.
However, manufacturers frequently use sintering aids—small amounts of other oxides like magnesia (MgO) or yttria (Y2O3). These additives can dramatically lower the required sintering temperature, saving energy and cost.
Particle Size
The starting particle size of the alumina powder is a critical factor. Finer powders have a much higher surface area, which provides more energy and contact points to drive the sintering process.
Therefore, components made from fine or nano-sized alumina powders can be sintered to high density at significantly lower temperatures than those made from coarser powders.
Dwell Time (Soaking Time)
Temperature and time are inextricably linked. You can often achieve a similar level of densification by firing at:
- A higher temperature for a shorter time.
- A lower temperature for a longer time.
The reference's example of 1350°C for 20 minutes highlights this relationship. Increasing that dwell time might allow for full density at an even lower temperature, while decreasing it would likely require a higher one.
Understanding the Trade-offs: Density vs. Grain Growth
Achieving the perfect alumina part is a balancing act. The most common trade-off you will face is maximizing density while minimizing unwanted grain growth.
The Pursuit of High Density
As discussed, the primary goal is usually to eliminate porosity and achieve a density as close to the theoretical maximum as possible. This is what gives technical ceramics their exceptional mechanical and electrical properties.
The Problem of Grain Growth
While heat drives densification, it also drives grain growth. At high temperatures, smaller crystal grains are consumed by larger ones.
If grains become too large, the material can actually become weaker and more prone to fracture. Over-firing—using too high a temperature or too long a dwell time—is a common cause of poor mechanical performance due to excessive grain growth.
Finding the Sweet Spot
The ultimate goal is to hold the material at a temperature just high enough, for just long enough, to achieve maximum densification before significant grain growth occurs. The parameters of 1350°C for 20 minutes to get 1.2 µm grains is an excellent example of a process optimized for this very outcome.
Choosing Your Firing Profile
There is no universal firing schedule. You must define your goal first, then design the process to meet it.
- If your primary focus is maximum density and fine grain structure: Use high-purity, sub-micron alumina powders and a carefully controlled cycle, potentially at a lower temperature (1350-1550°C) with a specific dwell time to prevent grain growth.
- If your primary focus is creating a porous structure (e.g., for a filter): Use lower temperatures or significantly shorter dwell times to intentionally stop the densification process early, preserving the voids between particles.
- If your primary focus is cost-effective, high-volume production: Use an alumina formulation that includes sintering aids, allowing you to fire at lower temperatures (1300-1450°C) and reduce energy consumption.
By understanding these variables, you can move from asking "what temperature" to designing the precise firing cycle your project requires.
Summary Table:
| Factor | Impact on Firing Temperature |
|---|---|
| Powder Purity | Higher purity often requires higher temperatures. |
| Sintering Aids | Additives like MgO can significantly lower temperature. |
| Particle Size | Finer powders enable sintering at lower temperatures. |
| Dwell Time | Longer times can compensate for lower temperatures. |
| Target Density | Maximum density requires precise temperature/time control. |
Ready to perfect your alumina sintering process?
At KINTEK, we specialize in providing the high-performance lab furnaces and expert support you need to achieve precise temperature control and optimal results. Whether you're working with high-purity alumina or cost-effective formulations, our equipment is designed for reliability and repeatability.
Contact our experts today to discuss your specific application and discover how KINTEK's solutions can enhance your laboratory's capabilities and drive your research forward.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- Why is a quartz tube furnace utilized in the thermal oxidation of MnCr2O4 coatings? Unlock Precise Selective Oxidation
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- What is a tubular furnace used for? Precision Heating for Material Synthesis & Analysis
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing
- How to clean a tube furnace? A Step-by-Step Guide for Safe and Effective Maintenance



















