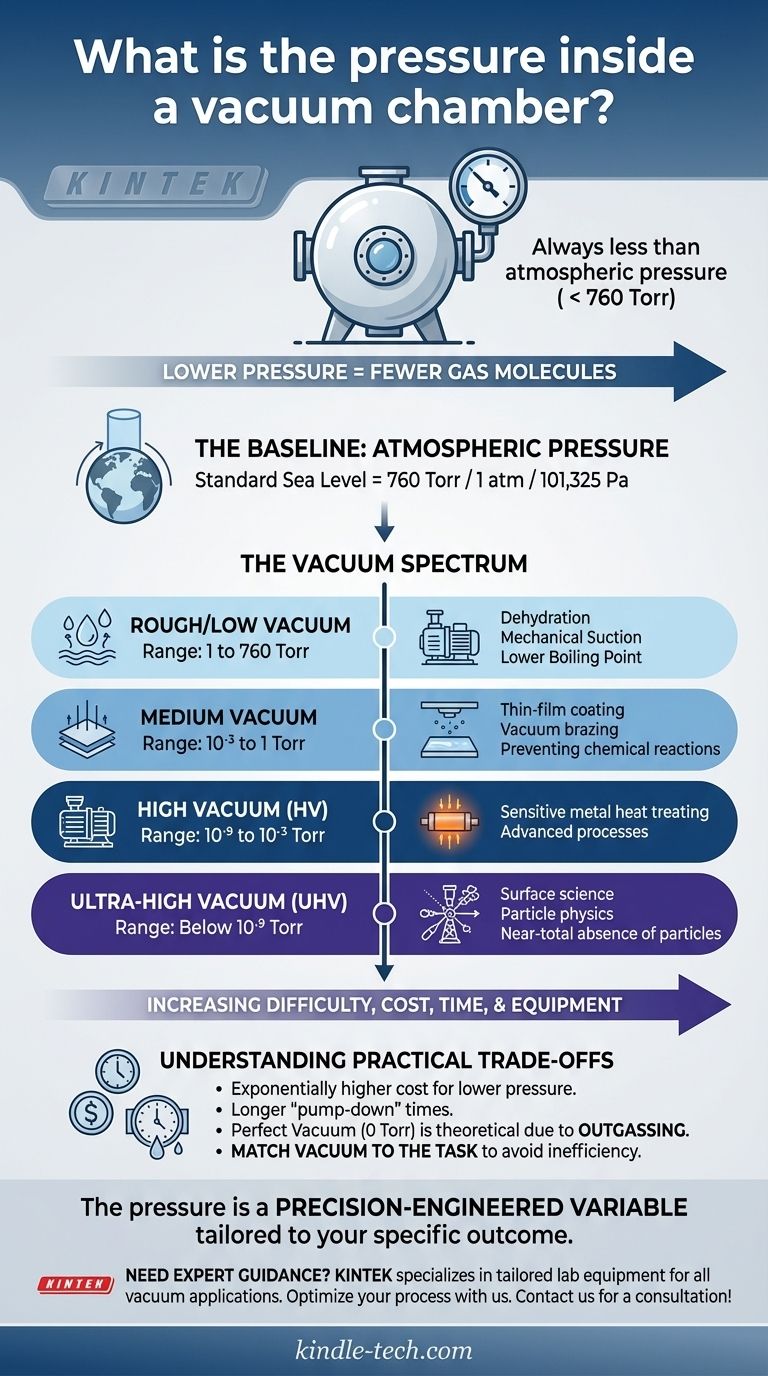
In short, the pressure inside a vacuum chamber is always less than the atmospheric pressure outside of it. This is not a single, fixed value but rather a controlled spectrum. A "vacuum" simply refers to any volume where the gas pressure has been reduced compared to the surrounding atmosphere, which at sea level is approximately 760 Torr (or 760 mmHg).
The key is to stop thinking of vacuum as "empty space" and start seeing it as a controlled, low-pressure environment. The specific pressure depends entirely on the application's requirements, ranging from a slight reduction below atmospheric pressure to the near-total absence of particles found in deep space.

The Concept of Pressure in a Vacuum
To understand the pressure inside a vacuum chamber, you must first establish a baseline: the air around us. This pressure is the starting point from which all vacuums are created.
Atmospheric Pressure: The "Zero" Point
Standard atmospheric pressure at sea level is the weight of the column of air above us. This is the pressure your vacuum pump must work against.
This baseline is defined as 1 atmosphere (atm), which is equivalent to approximately:
- 760 Torr
- 760 millimeters of mercury (mmHg)
- 101,325 Pascals (Pa)
Measuring the Absence of Pressure
Vacuum is the process of removing air molecules from a sealed chamber. Therefore, the pressure inside is measured on a scale from atmospheric pressure (760 Torr) down toward absolute zero pressure (0 Torr).
A lower pressure reading indicates fewer gas molecules and therefore a "higher" or "harder" vacuum.
The Different Levels of Vacuum
"Vacuum" is not a monolithic state. It is categorized into different qualities based on the residual pressure inside the chamber.
- Rough/Low Vacuum: 1 to 760 Torr
- Medium Vacuum: 10⁻³ to 1 Torr
- High Vacuum (HV): 10⁻⁹ to 10⁻³ Torr
- Ultra-High Vacuum (UHV): Below 10⁻⁹ Torr
Each level requires progressively more sophisticated and expensive equipment to achieve and maintain.
Understanding the Practical Trade-offs
Achieving the right vacuum level is a balancing act. Aiming for a lower pressure than necessary is a common and costly mistake.
The Cost of Lower Pressure
Reaching a high or ultra-high vacuum is exponentially more difficult than creating a rough vacuum. It demands advanced multi-stage pumps, specialized chamber materials, and significantly longer "pump-down" times.
The energy, time, and equipment costs increase dramatically as you try to remove the final remaining gas molecules.
Why a "Perfect Vacuum" is Impossible
A perfect vacuum, or absolute zero pressure (0 Torr), is a theoretical ideal, not a physical reality.
Even in the most advanced systems, gas molecules clinging to the chamber walls will slowly release in a process called outgassing. The chamber materials themselves can also sublimate or evaporate, contributing a tiny amount of pressure.
Matching the Vacuum to the Task
The goal is not to achieve the highest possible vacuum, but the appropriate vacuum for the application. Using a UHV system for a process that only requires a rough vacuum is like using a surgical scalpel to cut rope—inefficient and wasteful.
Making the Right Choice for Your Goal
The required pressure in your vacuum chamber is dictated entirely by your objective.
- If your primary focus is mechanical work or simple dehydration: A rough vacuum is often sufficient to provide suction force or to lower the boiling point of water.
- If your primary focus is preventing chemical reactions like oxidation: A medium or high vacuum is necessary for processes like thin-film coating, vacuum brazing, or heat treating sensitive metals.
- If your primary focus is surface science or particle physics: An ultra-high vacuum (UHV) is non-negotiable to ensure that particles can travel long distances without colliding with air molecules.
Ultimately, the pressure in a vacuum chamber is a precisely engineered variable, tailored to achieve a specific scientific or industrial outcome.
Summary Table:
| Vacuum Level | Pressure Range (Torr) | Common Applications |
|---|---|---|
| Rough/Low Vacuum | 1 to 760 | Dehydration, mechanical suction |
| Medium Vacuum | 10⁻³ to 1 | Thin-film coating, brazing |
| High Vacuum (HV) | 10⁻⁹ to 10⁻³ | Sensitive metal heat treating |
| Ultra-High Vacuum (UHV) | Below 10⁻⁹ | Surface science, particle physics |
Need Expert Guidance on Your Vacuum System?
Choosing the right vacuum level is critical for your process efficiency and cost-effectiveness. At KINTEK, we specialize in providing tailored lab equipment and consumables for all vacuum applications—from basic mechanical work to advanced surface science. Our experts can help you select the perfect vacuum chamber and pumping system to match your specific requirements, ensuring optimal performance without unnecessary expense.
Let's optimize your vacuum process together. Contact our team today for a personalized consultation!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- 304 316 Stainless Steel Vacuum Ball Valve Stop Valve for High Vacuum Systems
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
People Also Ask
- Why is a water circulating vacuum pump suitable for handling flammable or explosive gases? Inherent Safety Through Isothermal Compression
- How does a water circulating vacuum pump operate? Discover the Efficient Liquid Piston Principle
- What determines the vacuum degree achievable by a water circulating vacuum pump? Unlock the Physics of Its Limits
- What is the importance of a vacuum pump for Schottky hybrid interfaces? Achieve Atomic-Level Purity and Bonding
- What can I use a vacuum pump for? Powering Industrial Processes from Packaging to Automation