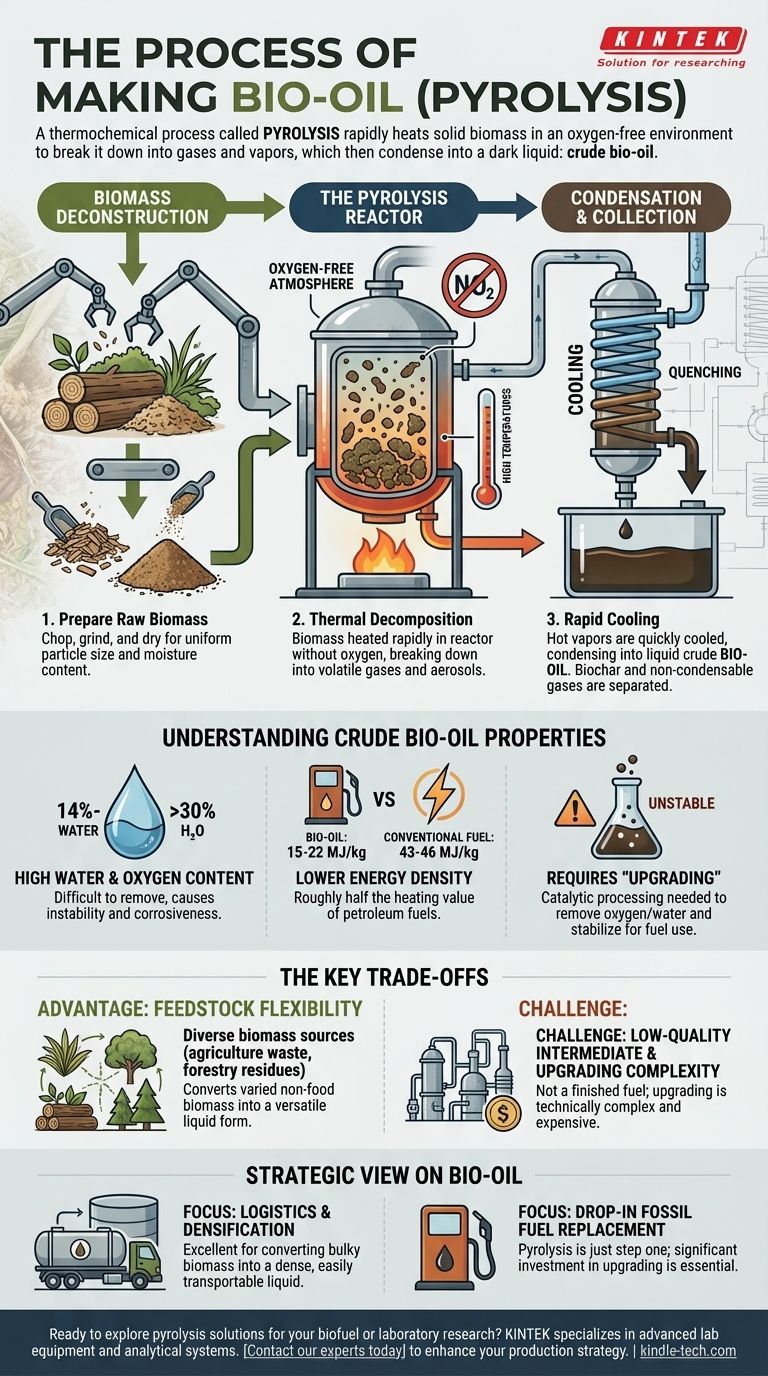
The primary method for producing bio-oil is a thermochemical process known as pyrolysis. This involves rapidly heating solid biomass, such as wood or agricultural waste, to high temperatures in an environment with no oxygen. The heat breaks the material down into gases and vapors, which are then quickly cooled and condensed into a dark, liquid "bio-oil," also called pyrolysis oil.
Pyrolysis efficiently converts solid biomass into a liquid intermediate, but this resulting bio-oil is not a finished fuel. Its high water and oxygen content give it fundamentally different properties from conventional petroleum, requiring significant further processing before it can be used.

The Core Process: From Biomass to Bio-Oil
The conversion of a solid plant material into a liquid fuel involves a multi-step thermal and chemical transformation. The central step is pyrolysis, which fundamentally alters the chemical structure of the biomass.
Step 1: Biomass Deconstruction
Before the main process can begin, the raw biomass feedstock must be prepared. This first step, known as deconstruction, involves breaking down the tough, rigid structure of the plant cell walls, typically by chopping, grinding, and drying the material.
This ensures the biomass has a consistent particle size and moisture content, allowing it to heat uniformly and react efficiently within the pyrolysis reactor.
Step 2: The Pyrolysis Reactor
The prepared biomass is fed into a reactor and heated to very high temperatures, all while in an oxygen-free atmosphere. The absence of oxygen is critical; it prevents the biomass from simply burning (combustion) and instead causes it to thermally decompose.
This decomposition breaks down large organic polymers like cellulose and lignin into smaller, volatile molecules, creating a hot mixture of gases and aerosols.
Step 3: Condensation and Collection
The hot gas and vapor stream is rapidly cooled, or "quenched." This sudden temperature drop causes the majority of these components to condense into a liquid.
This raw liquid is the crude bio-oil. Any non-condensable gases, along with a solid carbon-rich byproduct called biochar, are also separated and collected during this stage.
Understanding the Product: The Nature of Bio-Oil
It is crucial to understand that crude bio-oil is not a "drop-in" replacement for conventional fuel oil or crude petroleum. Its chemical properties make it a challenging intermediate product.
High Water and Oxygen Content
Bio-oil has a significant water content, typically ranging from 14% to over 30% by weight. This water is created during the pyrolysis reaction and is intimately mixed with the oil, making it difficult to remove through simple distillation.
Furthermore, bio-oil contains many oxygenated compounds, which are responsible for its instability and corrosiveness. This is the main reason for its lower energy content compared to hydrocarbons.
Lower Energy Density
The high concentration of water and oxygen gives bio-oil a much lower heating value than traditional fuels. Its energy density is typically 15–22 MJ/kg.
This is roughly half the energy density of conventional fuel oil, which is rated at 43–46 MJ/kg. An engine would need to burn approximately twice the volume of bio-oil to produce the same amount of energy.
The Need for "Upgrading"
Because of its instability, corrosiveness, and low energy value, crude bio-oil must be processed further in a stage called upgrading. This involves catalytic processes that remove oxygen and water, reduce its acidity, and combine smaller molecules into larger ones that are suitable for use as transportation fuel.
The Key Trade-offs
Bio-oil production via pyrolysis presents a clear set of advantages and disadvantages. Recognizing these trade-offs is essential for evaluating its role in the renewable energy sector.
Advantage: Feedstock Flexibility
Pyrolysis can convert a wide variety of non-food biomass—including agricultural residues, forestry waste, and dedicated energy crops—into a liquid form. This makes it a versatile pathway for utilizing otherwise low-value organic materials.
Challenge: A Low-Quality Intermediate
The direct output of pyrolysis is not a finished, ready-to-use fuel. Crude bio-oil is acidic, chemically unstable, and can degrade over time. Its properties require specialized handling and limit its direct applications.
Challenge: The Cost and Complexity of Upgrading
The upgrading processes required to turn crude bio-oil into a stable, high-energy fuel like renewable gasoline or diesel are technically complex and expensive. This second processing stage represents a significant hurdle to the economic viability of bio-oil as a large-scale fuel source.
How to View Bio-Oil in Your Strategy
Your approach to bio-oil should be dictated by your end goal. It is not a singular solution but an intermediate with specific applications.
- If your primary focus is logistics and energy densification: Pyrolysis is an excellent method for converting bulky, solid biomass into a dense liquid that is far cheaper and easier to transport and store.
- If your primary focus is creating a drop-in replacement for fossil fuels: You must view pyrolysis as only the first step. Your plan must include the significant technical and financial investment required for the subsequent upgrading process.
Ultimately, bio-oil represents a critical link in the chain of advanced biofuel production, offering a promising but challenging path from raw biomass to a finished fuel.
Summary Table:
| Process Stage | Key Action | Primary Output |
|---|---|---|
| 1. Biomass Deconstruction | Chopping, grinding, and drying raw biomass | Prepared, uniform feedstock |
| 2. Pyrolysis Reactor | Heating biomass in an oxygen-free environment | Hot mixture of gases and vapors |
| 3. Condensation & Collection | Rapidly cooling the vapor stream | Crude bio-oil, biochar, and gases |
Ready to explore pyrolysis solutions for your biofuel or laboratory research? KINTEK specializes in advanced lab equipment, including pyrolysis reactors and analytical systems, to help you efficiently convert biomass into bio-oil. Our expertise supports your R&D and process optimization needs. Contact our experts today to discuss how we can enhance your biofuel production strategy!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions



















