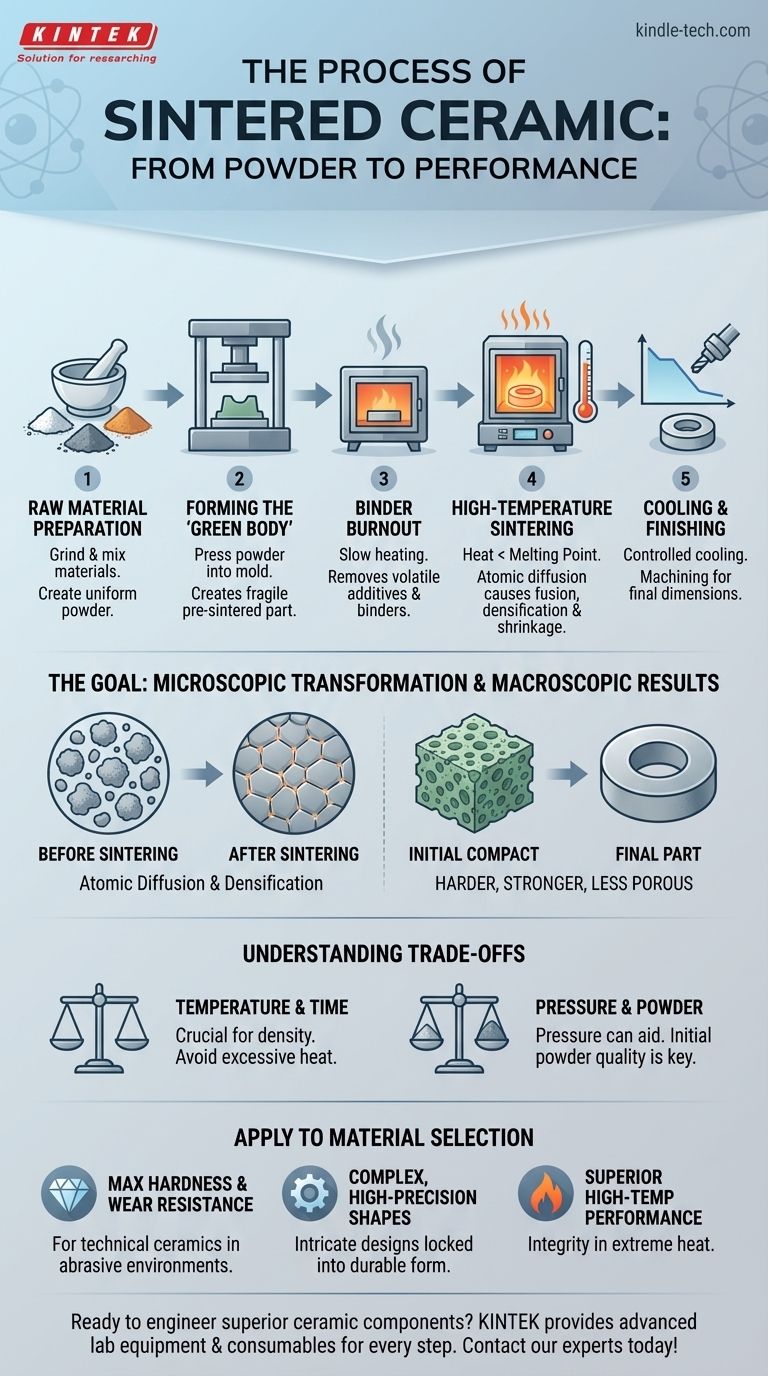
In short, the sintered ceramic process is a method of creating a dense, solid ceramic object from a powder without melting it. The core steps involve preparing a ceramic powder, pressing it into a desired shape (known as a "green body"), and then heating it to a high temperature below its melting point, which causes the individual particles to fuse together and compact the material.
Sintering is not simply baking; it is a fundamental material transformation. The process uses thermal energy to drive atomic diffusion, eliminating the empty spaces between powder particles and bonding them into a strong, monolithic component with significantly enhanced physical properties.

The Goal of Sintering: From Powder to Performance
The primary reason to sinter ceramics is to convert a fragile, porous powder compact into a robust, dense solid. This transformation is not just physical; it fundamentally re-engineers the material at a microscopic level.
The Microscopic Transformation
At high temperatures, the atoms at the contact points between ceramic particles become mobile. They begin to diffuse, or migrate, across the boundaries of adjacent particles.
This atomic movement closes the pores and voids between the particles, pulling them tightly together. As this happens, the grain boundaries shift and grow, effectively fusing the once-separate particles into a unified, polycrystalline structure.
The Macroscopic Results
This microscopic densification results in dramatic and desirable changes to the material's bulk properties. The final sintered part is significantly harder, stronger, and less porous than the initial powder compact.
For certain materials, like zirconia, sintering also induces a phase transformation in its crystalline structure, which is directly responsible for its exceptional strength and fracture toughness.
A Step-by-Step Breakdown of the Process
While specific parameters vary by material, the manufacturing of a sintered ceramic component follows a well-defined sequence.
Step 1: Raw Material Preparation
The process begins with natural raw materials like silica, clay, feldspar, or synthetic powders like alumina and zirconia. These materials are ground into fine, uniform particles.
Often, these powders are mixed with water, a binder, and other agents to form a liquid slurry. This slurry may then be spray-dried to create uniform, free-flowing granules perfectly suited for the next step.
Step 2: Forming the "Green Body"
The prepared ceramic powder is compacted into the desired shape. This pre-sintered, fragile part is called a "green body."
The most common forming method is pressing the powder into a mold or die under high pressure. This creates a coherent shape that is strong enough to be handled.
Step 3: Binder Burnout (Pre-Sintering)
The green body is heated slowly to a relatively low temperature. The purpose of this stage is to carefully burn off the binder and any other volatile additives that were used during the preparation and forming stages.
Step 4: High-Temperature Sintering
This is the core of the process. The part is heated in a kiln to a very high temperature, often exceeding 1200°C, but critically, this is below the material's melting point.
During this stage, the atomic diffusion and particle fusion occur, causing the part to densify and shrink significantly. The temperature and duration are precisely controlled to achieve the desired final density and grain structure.
Step 5: Cooling and Finishing
After sintering, the component is cooled in a controlled manner to prevent thermal shock and cracking. The final part is extremely hard and may require finishing.
Because of their hardness, sintered ceramics must be machined with specialized equipment, such as diamond grinding tools or ultrasonic machining. Some parts may also be metallized for brazing and assembly with other components.
Understanding the Trade-offs
Sintering is a powerful process, but its success depends on careful control of key variables.
Temperature and Time are Critical
The relationship between temperature, time, and final density is crucial. Insufficient heat or time will result in a porous, weak part. Conversely, excessive heat can cause abnormal grain growth, which can paradoxically reduce the material's strength and toughness.
Pressure as a Variable
While most sintering relies on heat alone, pressure can also be applied during the heating cycle (a process known as hot pressing). This allows for densification at lower temperatures and can result in superior properties, though it is a more complex and costly technique.
The Importance of the Starting Powder
The final quality of a sintered ceramic is highly dependent on the initial powder. The size, shape, and uniformity of the starting particles directly impact how efficiently the part will densify, influencing the properties of the finished component.
How to Apply This to Your Material Selection
Understanding the fundamentals of sintering helps you make better decisions when specifying materials for a given application.
- If your primary focus is maximum hardness and wear resistance: Sintering is the essential process for creating technical ceramics like alumina, silicon carbide, and zirconia that outperform metals in abrasive environments.
- If your primary focus is complex shapes with high precision: The "green body" forming stage allows for intricate designs via molding or pressing, which are then locked into a durable, stable form by the sintering process.
- If your primary focus is high-temperature performance: Sintered ceramics maintain their structural integrity and strength at temperatures where most metals would fail, making them ideal for furnace components, engine parts, and aerospace applications.
Ultimately, understanding the sintering process transforms it from a simple "firing" step into a highly controlled method for engineering advanced materials with specific, superior properties.
Summary Table:
| Step | Key Action | Outcome |
|---|---|---|
| 1. Powder Prep | Grind & mix raw materials | Uniform, fine powder |
| 2. Forming | Press powder into a mold | Creates a fragile 'green body' |
| 3. Binder Burnout | Heat to low temperature | Removes additives and binders |
| 4. Sintering | Heat to high temperature (below melting point) | Particles fuse; part densifies and shrinks |
| 5. Finishing | Controlled cooling & machining | Final, high-performance ceramic part |
Ready to engineer superior ceramic components?
The sintering process is fundamental to creating high-performance materials, but achieving optimal results requires precise control and the right equipment. KINTEK specializes in providing the advanced lab equipment and consumables needed for every step of the ceramic sintering process, from powder preparation to high-temperature furnaces.
We help our laboratory customers achieve:
- Maximum Hardness & Wear Resistance: Create technical ceramics that outperform metals.
- Complex, High-Precision Shapes: Utilize forming and sintering for intricate designs.
- Superior High-Temperature Performance: Develop components that maintain integrity in extreme conditions.
Let's discuss how our sintering solutions can enhance your material development. Contact our experts today to find the right equipment for your specific ceramic needs.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the difference between sintering and firing? A Guide to Thermal Process Terminology
- What is the difference between sintering and vitrification? Key Thermal Process Distinctions
- What are the uses of furnace in chemistry laboratory? Unlock High-Temperature Material Synthesis and Analysis
- What are the lab safety rules for heating substances? Essential Protocols to Prevent Accidents
- What is muffle in muffle furnace? The Key to Contamination-Free High-Temperature Processing



















