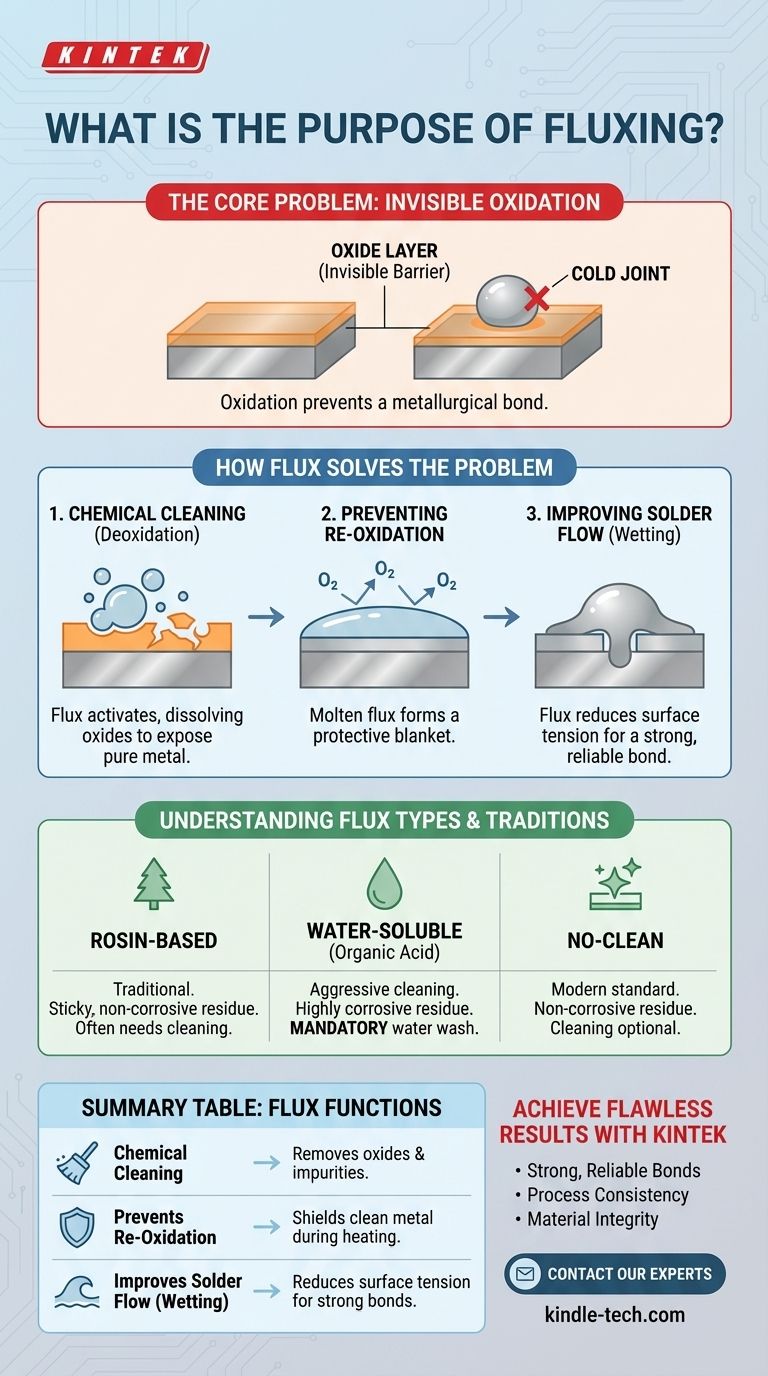
The primary purpose of flux is to act as a chemical cleaning agent. It prepares metal surfaces for soldering by removing invisible oxides and impurities. This cleaning action is absolutely critical for creating a strong, reliable metallurgical bond between the solder and the metal components.
The core problem is that solder cannot bond to oxidized metal. Flux solves this by chemically stripping away the oxide layer and then shielding the clean metal from the air, allowing the solder to flow properly and form a true chemical connection.

The Core Problem: Invisible Oxidation
What is Oxidation?
Nearly all metals react with oxygen in the air. This chemical reaction forms a very thin, often invisible, layer of metal oxide on the surface.
This process is similar to how iron rusts, but it happens on soldering surfaces like copper and tin far more subtly. Even a brand-new, shiny circuit board pad has an oxide layer.
Why Oxides Prevent Good Solder Joints
Solder does not just physically "glue" parts together; it forms a metallurgical bond. This requires direct, atom-to-atom contact between the solder alloy and the base metals.
An oxide layer acts as a barrier, preventing this essential contact. Attempting to solder an oxidized surface will cause the molten solder to "bead up" and refuse to flow, resulting in a weak, unreliable connection known as a cold joint.
How Flux Solves the Oxidation Problem
Flux is a multi-step chemical tool that activates with the heat from a soldering iron. It performs three critical functions in rapid succession.
Step 1: Chemical Cleaning (Deoxidation)
As the flux is heated, it becomes chemically active. Its acidic properties dissolve and break down the existing metal oxides on the component leads and pads. This exposes the pure, clean base metal underneath, which is essential for a proper bond.
Step 2: Preventing Re-Oxidation
The intense heat required for soldering dramatically accelerates the rate of oxidation. Once the flux has cleaned the surface, the molten flux itself creates a protective blanket.
This liquid shield prevents oxygen from reaching the freshly cleaned metal, keeping it pure and solderable for the few seconds needed to complete the joint.
Step 3: Improving Solder Flow (Wetting)
Finally, flux acts as a surfactant, which reduces the surface tension of the molten solder. This allows the solder to flow smoothly and evenly across the metal surfaces.
This phenomenon, known as wetting, is the hallmark of a good solder joint. It allows the solder to penetrate small gaps through capillary action, creating a strong and electrically conductive connection.
Understanding the Trade-offs and Flux Types
Not all fluxes are the same. The choice depends on the application, the metals being joined, and the requirements for cleaning after soldering.
Rosin-Based Flux
Derived from pine tree resin, this is a traditional choice for electronics. It is not very corrosive but leaves behind a sticky residue that can be insulating. For critical applications, this residue is typically cleaned off with a solvent.
Water-Soluble (Organic Acid) Flux
This is a more chemically aggressive and powerful type of flux. It is excellent at cleaning surfaces but its residue is highly corrosive and conductive. It is mandatory that this residue be thoroughly cleaned with deionized water after soldering to prevent long-term damage to the circuit.
No-Clean Flux
This is the most common type used in modern electronics manufacturing. It is engineered to have a residue that is non-corrosive and non-conductive after soldering. While cleaning is not strictly required, many high-reliability applications still involve a cleaning process to ensure perfect performance.
Making the Right Choice for Your Task
Choosing the correct flux is essential for achieving a successful outcome. Your decision should be based on your specific goal.
- If your primary focus is hobbyist electronics or repair: Use a solder wire with a rosin or no-clean flux core. This is the simplest and safest option for general-purpose work.
- If your primary focus is professional electronics manufacturing: Follow your process specifications, which will likely call for a specific no-clean or water-soluble flux designed for high-volume production and cleaning systems.
- If your primary focus is plumbing or structural metalwork: Use an aggressive, acid-based flux specifically formulated for joining copper pipes or other structural metals, as these require much stronger deoxidation.
Ultimately, flux is the unsung hero that makes reliable soldering possible.
Summary Table:
| Flux Function | Key Benefit |
|---|---|
| Chemical Cleaning | Removes invisible oxides and impurities from metal surfaces. |
| Prevents Re-Oxidation | Shields clean metal from air during heating, maintaining solderability. |
| Improves Solder Flow (Wetting) | Reduces surface tension for smooth, even solder coverage and strong bonds. |
Achieve Flawless Soldering Results with KINTEK
Struggling with weak solder joints or poor wetting? The right flux is critical for success. KINTEK specializes in high-purity laboratory equipment and consumables, providing reliable solutions for all your soldering and materials joining needs.
Our products help ensure:
- Strong, Reliable Bonds: Eliminate cold joints and ensure metallurgical connections.
- Process Consistency: Achieve repeatable results in R&D, prototyping, or production.
- Material Integrity: Protect your components with the appropriate flux chemistry.
Whether you're in electronics manufacturing, research, or repair, KINTEK has the expertise and products to support your work. Contact our experts today to discuss your specific application and find the perfect flux solution for your lab!
Visual Guide
Related Products
- Copper Foam
- High-Purity Titanium Foil and Sheet for Industrial Applications
- High Purity Zinc Foil for Battery Lab Applications
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- What are the characteristics of copper foam? Unlock High-Performance Thermal and Electrical Solutions
- What are the common applications of copper foam? A Guide to Its High-Performance Uses
- How can different materials have different heat capacity? Unlocking the Microscopic Secrets of Energy Storage
- What are the factors that affect heat transfer? Master the Key Variables for Optimal Thermal Performance
- What are the proper storage conditions for nickel and copper foam? A Guide to Preserving Performance



















