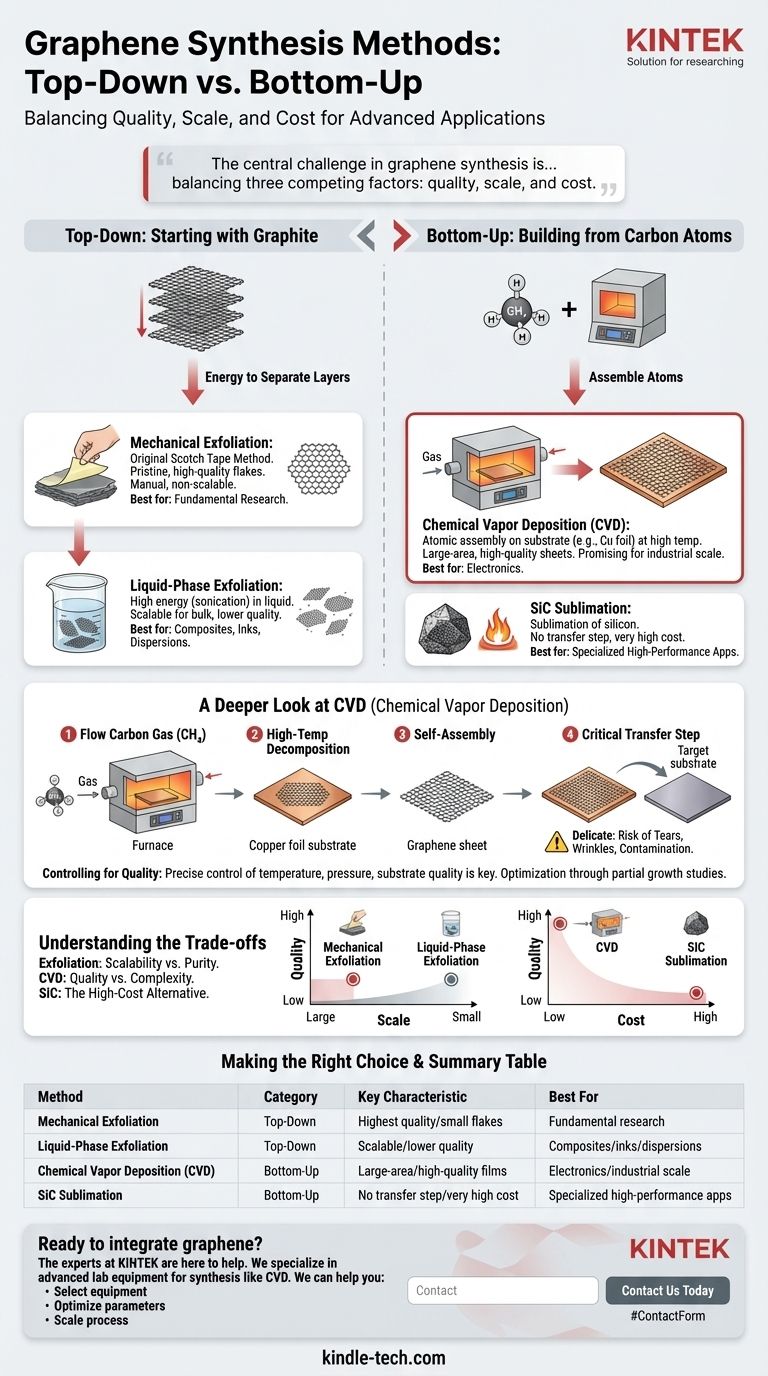
In essence, all graphene synthesis techniques fall into two fundamental categories: top-down methods that start with graphite and break it down, and bottom-up methods that build graphene from individual carbon atoms. While many variations exist, the most important method for producing high-quality, large-area graphene suitable for electronics is Chemical Vapor Deposition (CVD), a bottom-up approach.
The central challenge in graphene synthesis is not simply creating it, but balancing three competing factors: quality, scale, and cost. While simple exfoliation can produce pristine flakes for research, only methods like CVD can produce the large, uniform sheets required for advanced applications.
The Two Core Philosophies: Top-Down vs. Bottom-Up
Every synthesis method begins with one of two distinct starting points. Understanding this division is the first step to grasping the trade-offs involved.
Top-Down: Starting with Graphite
This approach is fundamentally destructive. You begin with bulk graphite—essentially a stack of countless graphene layers—and use energy to separate those layers.
The most famous top-down method is mechanical exfoliation. This is the original "Scotch tape" method, where adhesive tape peels layers from a graphite crystal. It produces exceptionally high-quality, defect-free graphene flakes.
However, mechanical exfoliation is not scalable and is therefore limited to fundamental research.
Another common method is liquid-phase exfoliation. In this process, graphite is submerged in a liquid and subjected to high energy (like sonication) to break the layers apart. This is better for mass production but often results in smaller flakes with lower electrical quality.
Bottom-Up: Building from Carbon Atoms
This approach is fundamentally constructive. You start with a source of carbon atoms—typically a gas—and assemble them into a single, continuous sheet of graphene on a substrate.
The dominant bottom-up method is Chemical Vapor Deposition (CVD). It is widely considered the most promising technique for industrial-scale production of high-quality graphene.
Other bottom-up methods exist, such as the sublimation of silicon carbide (SiC) or arc discharge, but CVD offers the best balance of quality and scalability for most applications.
A Deeper Look at Chemical Vapor Deposition (CVD)
Because of its importance for next-generation electronics, the CVD process warrants a closer look. It is a highly controlled process of atomic assembly.
How CVD Works
The process involves flowing a carbon-containing gas, most commonly methane (CH₄), into a high-temperature furnace.
Inside the furnace is a metal substrate, typically a thin copper (Cu) foil. At high temperatures, the methane decomposes, and carbon atoms deposit onto the surface of the copper, self-assembling into a continuous, single-atom-thick sheet of graphene.
The Critical Transfer Step
The graphene grown on the copper foil must then be transferred to a target substrate (like silicon or flexible plastic) for use in a device. This transfer process is delicate and can introduce tears, wrinkles, or contamination, which remains a significant engineering challenge.
Controlling for Quality
The final quality of the graphene film depends heavily on precise control over synthesis parameters. Factors like temperature, gas pressure, and the quality of the substrate all influence the final product.
Researchers use techniques like "partial growth studies"—stopping the process before a full film forms—to study how individual graphene crystals nucleate and grow. This helps them optimize conditions to minimize defects and create a more perfect film.
Understanding the Trade-offs
No single synthesis method is perfect; each comes with inherent compromises.
Exfoliation: Scalability vs. Purity
Mechanical exfoliation provides the purest form of graphene, but it is a manual process that produces tiny, randomly placed flakes. It is impossible to scale for manufacturing. Liquid-phase exfoliation is scalable for bulk materials like inks or composites, but the resulting flakes are less pristine.
CVD: Quality vs. Complexity
CVD produces the large-area, high-quality films needed for electronics. However, it requires expensive, specialized equipment, high temperatures, and a complex transfer step that can compromise the final quality and increase cost.
SiC Sublimation: The High-Cost Alternative
Heating silicon carbide to extreme temperatures causes silicon to sublimate away, leaving a layer of graphene directly on the wafer. This avoids a transfer step but is prohibitively expensive for all but the most specialized, high-performance applications.
Making the Right Choice for Your Goal
The best synthesis method is entirely dependent on your end-use application.
- If your primary focus is fundamental research: Mechanical exfoliation provides the highest-quality, defect-free flakes for lab-scale experiments.
- If your primary focus is large-area electronics: Chemical Vapor Deposition (CVD) is the most promising method for producing high-quality, continuous graphene films.
- If your primary focus is creating composites, inks, or dispersions: Liquid-phase exfoliation is a cost-effective method for mass-producing graphene flakes where pristine electrical properties are not the top priority.
Ultimately, the ideal synthesis method is dictated by the specific balance of quality, scale, and cost your application demands.
Summary Table:
| Method | Category | Key Characteristic | Best For |
|---|---|---|---|
| Mechanical Exfoliation | Top-Down | Highest quality, small flakes | Fundamental research |
| Liquid-Phase Exfoliation | Top-Down | Scalable, lower quality | Composites, inks, dispersions |
| Chemical Vapor Deposition (CVD) | Bottom-Up | Large-area, high-quality films | Electronics, industrial scale |
| SiC Sublimation | Bottom-Up | No transfer step, very high cost | Specialized high-performance applications |
Ready to integrate graphene into your research or product development?
Choosing the right synthesis method is critical to achieving your goals for quality, scale, and budget. The experts at KINTEK are here to help. We specialize in providing the advanced lab equipment and consumables needed for cutting-edge material synthesis, including processes like CVD.
We can help you:
- Select the right equipment for your specific graphene application.
- Optimize your synthesis parameters for superior results.
- Scale your process from research to production.
Contact us today using the form below to discuss how our solutions can accelerate your innovation with graphene and other advanced materials.
Visual Guide
Related Products
- CVD Diamond Cutting Tool Blanks for Precision Machining
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
- Carbon Graphite Plate Manufactured by Isostatic Pressing Method
- Lab Sterile Slapping Type Homogenizer for Tissue Mashing and Dispersing
People Also Ask
- What is the significance of the ultra-high pressure in solid-state battery assembly? Achieve Atomic-Level Contact
- How is a galvanostat used to evaluate F-rGO layers? Quantifying Protective Performance in Lithium Metal Batteries
- What is the purpose of boric acid treatment in graphite anode repair? Enhance Anode Performance and Structural Integrity
- How do liquid nitrogen and vacuum equipment contribute to safety? Expert Battery Discharge Protocols
- What is the primary function of a vacuum drying oven in LiFePO4 cathode preparation? Ensure High Battery Performance
- What role does a vacuum drying oven play in SPE and cathode preparation? Ensure Battery Purity & Performance
- What are the requirements for CuBi2O4 composite coatings vs. copper? Optimize Deposition with Precision Control
- Why must the drying of PEO-TPP composite layers be in an argon glove box? Ensure Peak Battery Performance



















