In short, the sample holder in an electrochemical experiment typically functions as the working electrode. This dual role is fundamental to the entire setup, as it is responsible for both physically securing the material being studied and serving as the primary site where the electrochemical reaction of interest takes place.
The sample holder's critical function is to act as the working electrode, providing the necessary mechanical support for the sample while simultaneously making the electrical connection needed to control the experiment and measure the reaction.

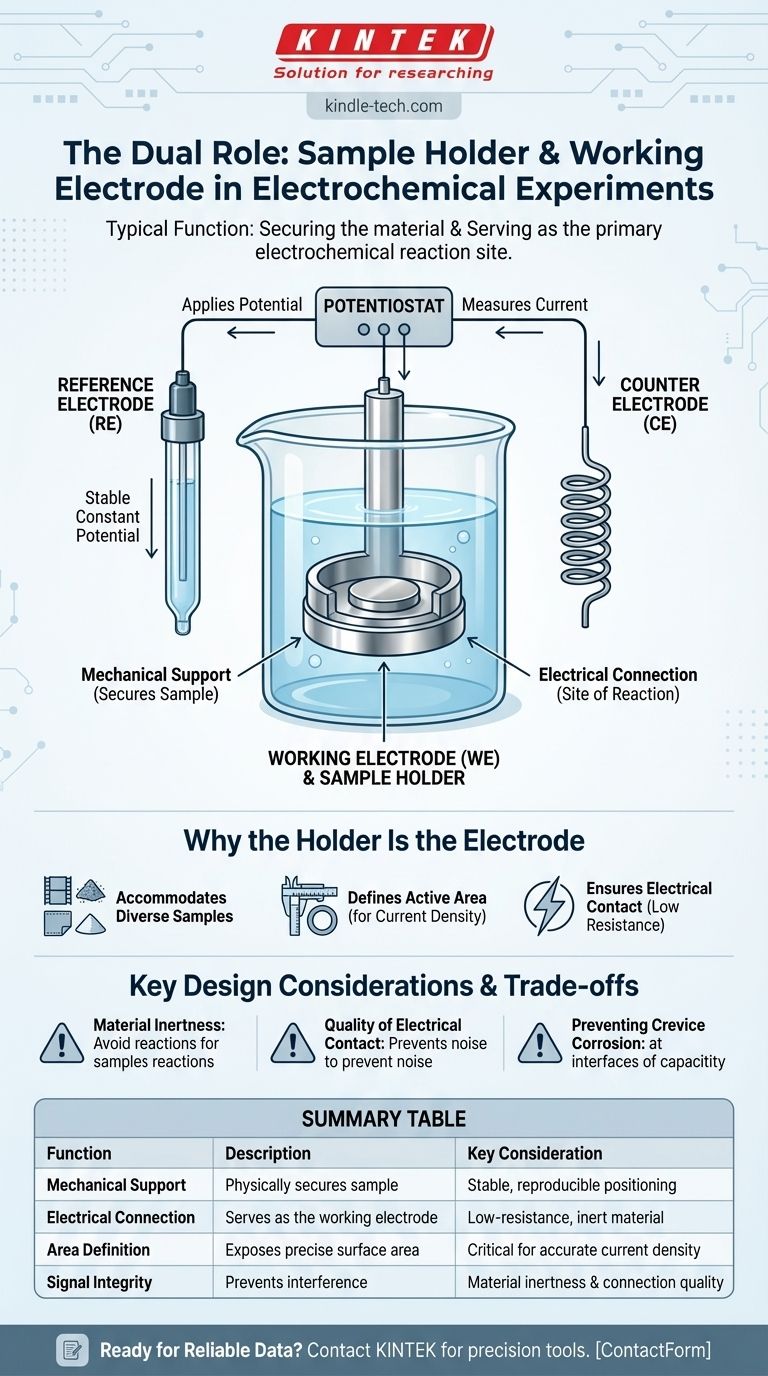
The Role of the Working Electrode
To understand the sample holder's function, one must first understand the role of the working electrode (WE) in a standard three-electrode electrochemical cell.
The Heart of the Experiment
The working electrode is the centerpiece of the measurement. It is the electrode at which the specific chemical reaction being investigated—either oxidation or reduction—occurs.
All measurements of potential and current are made relative to the processes happening at this surface.
A Key Part of the Three-Electrode System
Electrochemistry typically uses a three-electrode system to decouple different functions and achieve precise control.
The working electrode is the surface of interest. The reference electrode provides a stable, constant potential to measure against. The counter electrode acts to complete the electrical circuit, allowing current to flow without affecting the potential of the reference electrode.
Applying Potential and Measuring Current
A device called a potentiostat controls the potential difference between the working electrode and the reference electrode.
Simultaneously, it measures the resulting current that flows between the working electrode and the counter electrode. This current is directly proportional to the rate of the electrochemical reaction occurring on your sample.
Why the Holder Is the Electrode
The design of a sample holder as the working electrode is a practical solution for studying a vast range of materials that cannot easily be formed into a simple wire or rod.
Accommodating Diverse Samples
Many materials of interest—such as thin films, catalysts deposited as powders, corroding metal coupons, or biological membranes—require a specialized holder.
The holder provides the necessary mechanical stability and electrical connection that the sample itself cannot offer.
Defining the Active Area
A crucial function of the holder is to expose a precise and known surface area of the sample to the electrolyte solution.
This is often achieved using O-rings or non-conductive masks that isolate a specific geometric area. Knowing this area is essential for calculating current density (Amps/cm²), a fundamental metric for comparing results.
Ensuring Electrical Contact
The holder is constructed from a conductive material, such as stainless steel, platinum, or glassy carbon.
This ensures a reliable, low-resistance electrical path from the sample to the potentiostat's measurement leads.
Key Design Considerations and Trade-offs
The choice and design of a sample holder are not trivial and involve critical trade-offs that can directly impact the quality of your data.
Material Inertness
The material of the holder itself must be chemically inert within the potential window and electrolyte being used.
If the holder reacts, corrodes, or undergoes its own electrochemical reactions, it will generate an interfering signal that corrupts the data from your actual sample.
Quality of Electrical Contact
A poor electrical connection between the sample and the holder is a frequent source of experimental error.
This can introduce significant noise (unwanted fluctuations) into your measurements or lead to inaccurate control of the applied potential, invalidating the results.
Preventing Crevice Corrosion
The small gap at the interface between the sample, the holder, and the O-ring can be susceptible to crevice corrosion.
This localized form of corrosion can damage the sample and produce electrochemical signals that do not represent the bulk material's behavior, leading to misinterpretation of the data.
Making the Right Choice for Your Goal
The optimal sample holder design depends entirely on the objective of your experiment.
- If your primary focus is corrosion analysis: Your priority is a holder made of a highly resistant alloy that creates a watertight seal, ensuring you are only measuring the corrosion behavior of your exposed sample coupon.
- If your primary focus is electrocatalyst testing: You need a holder with a well-defined, polished, and inert conductive surface (like glassy carbon) onto which you can reproducibly deposit your catalyst ink.
- If your primary focus is battery material development: You will use a specialized cell holder (like a coin cell) that applies consistent pressure to ensure good contact between your electrode material, separator, and current collectors.
Ultimately, recognizing that your sample holder is an active and critical electrical component is the first step toward collecting reliable and meaningful electrochemical data.
Summary Table:
| Function | Description | Key Consideration |
|---|---|---|
| Mechanical Support | Physically secures the sample (e.g., film, powder, coupon). | Must ensure stable, reproducible positioning. |
| Electrical Connection | Serves as the working electrode, the site of the reaction. | Requires low-resistance, inert conductive material. |
| Area Definition | Exposes a precise, known surface area of the sample to the electrolyte. | Critical for calculating accurate current density. |
| Signal Integrity | Prevents interference from holder corrosion or poor contacts. | Material inertness and connection quality are paramount. |
Ready to achieve reliable electrochemical data? The right sample holder is critical for success. KINTEK specializes in high-quality lab equipment and consumables for all your electrochemical needs. Whether you require corrosion-resistant holders, polished conductive surfaces for catalyst testing, or specialized cell designs, we provide the precision tools to ensure accurate results.
Contact our experts today to discuss your specific application and discover how our solutions can enhance your laboratory's performance and data integrity.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
People Also Ask
- What role does a water-jacketed electrolytic cell play in variable-temperature electrochemical corrosion measurements?
- How is a three-electrode electrochemical electrolytic cell utilized to evaluate Zr-Nb alloy corrosion resistance?
- What type of electrode system is the coating evaluation electrolytic cell designed for? Unlock Precise Coating Analysis
- What is the operating principle of a flat plate corrosion electrolytic cell? A Guide to Controlled Materials Testing
- How does a three-electrode electrolytic cell function? Precision Testing for 8620 Steel in Corrosive Environments



















