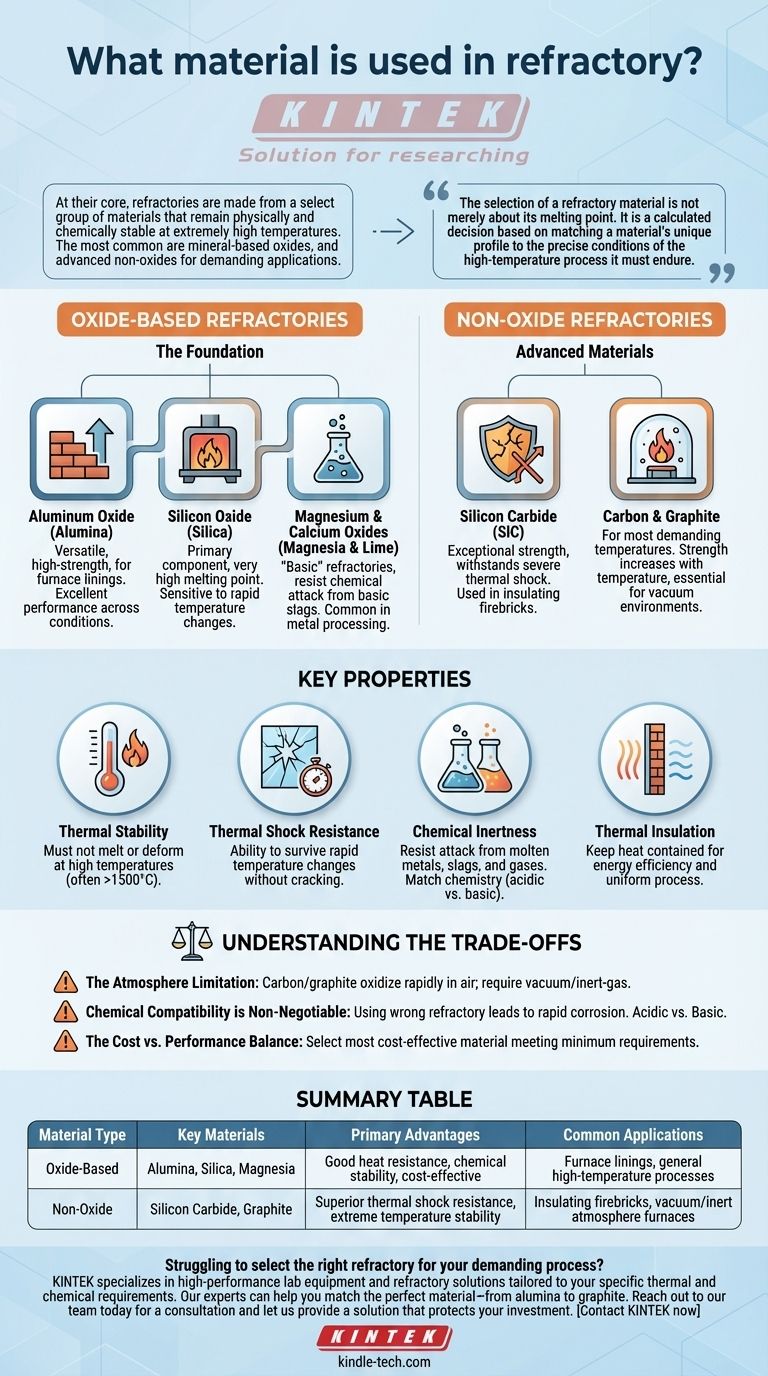
At their core, refractories are made from a select group of materials that remain physically and chemically stable at extremely high temperatures. The most common are mineral-based oxides, including aluminum oxide (alumina), silicon oxide (silica), and magnesium oxide (magnesia). For more demanding applications, advanced non-oxide materials like silicon carbide and carbon-based graphite are also used.
The selection of a refractory material is not merely about its melting point. It is a calculated decision based on matching a material's unique profile—its chemical inertness, thermal shock resistance, and strength—to the precise conditions of the high-temperature process it must endure.

The Foundation: Oxide-Based Refractories
The vast majority of refractory applications rely on oxide ceramics. These materials offer a robust combination of heat resistance and chemical stability at a reasonable cost.
Aluminum Oxide (Alumina)
Alumina (Al₂O₃) is a versatile, high-strength workhorse in the world of refractories. It is widely used in furnace linings and is known for its excellent performance across a range of conditions.
Silicon Oxide (Silica)
Silica (SiO₂) is a primary component of many common refractories, especially fire clays. While it has a very high melting point, its performance can be sensitive to rapid temperature changes.
Magnesium & Calcium Oxides (Magnesia & Lime)
These materials are classified as "basic" refractories. They are chosen specifically for their ability to resist chemical attack from basic slags and environments, which are common in steel and metal processing.
Advanced Materials: Non-Oxide Refractories
When conditions become more extreme, either in temperature, thermal cycling, or chemical environment, non-oxide materials provide enhanced performance.
Silicon Carbide (SiC)
Known for its exceptional strength and stability, silicon carbide is often used in insulating firebricks. Its primary advantage is its ability to withstand severe thermal shock—rapid heating and cooling—without cracking.
Carbon & Graphite
Carbon, particularly in the form of high-purity graphite, is used in the most demanding temperature environments. Its strength actually increases with temperature, and it is essential for heat shields and even heating elements in controlled-atmosphere furnaces.
Understanding the Key Properties
Choosing the right material requires understanding why these materials are selected. The decision hinges on a few critical properties beyond simple heat resistance.
Thermal Stability
This is the fundamental requirement. The material must not melt, soften, or deform at the operating temperature, which can often exceed 1500°C (2732°F).
Thermal Shock Resistance
This measures the material's ability to survive rapid temperature changes. Materials like graphite and silicon carbide excel here, while silica-based refractories can be more susceptible to cracking.
Chemical Inertness
A refractory must resist chemical attack from the substances it contains, such as molten metal, slag, or process gases. An acidic refractory like silica will quickly degrade in a basic environment, and vice versa.
Thermal Insulation
Some refractories, like carbon felt, are designed to be excellent insulators to keep heat contained. Others may need to be more conductive. This property is critical for ensuring uniform process conditions and energy efficiency.
Understanding the Trade-offs
No single refractory material is perfect for every application. The choice always involves balancing performance against limitations and cost.
The Atmosphere Limitation
Carbon and graphite are exceptional at high temperatures, but they will rapidly oxidize and burn away in the presence of oxygen (air). Their use is restricted to vacuum or inert-gas atmospheres.
Chemical Compatibility is Non-Negotiable
Using the wrong type of refractory for your chemical environment is a primary cause of failure. An acidic refractory (silica-based) used with a basic slag (high in lime or magnesia) will result in rapid corrosion and breakdown of the lining.
The Cost vs. Performance Balance
Simple fire clay bricks are significantly less expensive than high-purity, engineered silicon carbide or alumina shapes. The goal is to select the most cost-effective material that meets the minimum performance requirements for the life of the furnace.
Choosing the Right Refractory for Your Application
Your final choice depends entirely on the specific demands of your process.
- If your primary focus is general-purpose heating in air: Alumina or alumina-silica firebricks provide the best balance of performance and cost.
- If your process involves rapid heating and cooling cycles: Prioritize materials with excellent thermal shock resistance, such as silicon carbide.
- If you are containing aggressive molten metals or slags: You must match the refractory's chemistry (acidic vs. basic) to the process chemistry to prevent corrosion.
- If you need to reach extreme temperatures in a vacuum or inert atmosphere: High-purity graphite is often the superior choice for its unmatched thermal stability.
Ultimately, the most durable refractory is the one best matched to its specific operational demands.
Summary Table:
| Material Type | Key Materials | Primary Advantages | Common Applications |
|---|---|---|---|
| Oxide-Based | Alumina (Al₂O₃), Silica (SiO₂), Magnesia (MgO) | Good heat resistance, chemical stability, cost-effective | Furnace linings, general high-temperature processes |
| Non-Oxide | Silicon Carbide (SiC), Graphite (C) | Superior thermal shock resistance, extreme temperature stability | Insulating firebricks, vacuum/inert atmosphere furnaces |
Struggling to select the right refractory for your demanding process? The wrong choice can lead to equipment failure, safety risks, and costly downtime. KINTEK specializes in high-performance lab equipment and consumables, including refractory solutions tailored to your specific thermal and chemical requirements. Our experts can help you match the perfect material—from alumina to graphite—to ensure durability, efficiency, and safety in your laboratory. Reach out to our team today for a consultation and let us provide a solution that protects your investment. Contact KINTEK now to get started!
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What is the function of a vacuum filtration system? Enhance Photovoltaic Metal Recovery with 0.45µm Precision
- Why is tantalum foil required when using graphite molds for sintering yttrium oxide? Ensure Optical Purity
- How does high-speed stirring equipment contribute to the uniformity of zinc borate suspensions? Achieve Pure Synthesis
- Why are zirconia grinding balls preferred for NiCrAlY-Mo-Ag powders? Ensure Maximum Purity and Durability
- What roles do graphite foil and boron nitride plates play in LLZO ultra-fast sintering? Optimize Solid-State Electrolytes
- What material is a heat treatment basket made of? Choose the Right Alloy for Your Furnace
- Why are zirconia grinding balls preferred for LLZTO milling? Ensure Material Purity & High Ionic Conductivity
- What is the role of a two-stage rotary vane vacuum pump in magnesium alloy sublimation? Enhance Efficiency and Purity



















