Poor current flow in a platinum mesh electrode is a common issue that typically points to one of two primary causes: a faulty electrical connection or a contaminated electrode surface. The first step is always to verify that all physical connections are secure and corrosion-free. If the connections are sound, the next step is to clean the electrode's active surface to remove any blockages or adsorbed species that are impeding the electrochemical reaction.
Poor current flow is a symptom, not the root cause. It signals a breakdown in either the external electrical circuit or the internal electrochemical interface, requiring a systematic check of connections, surface cleanliness, and potential material degradation.

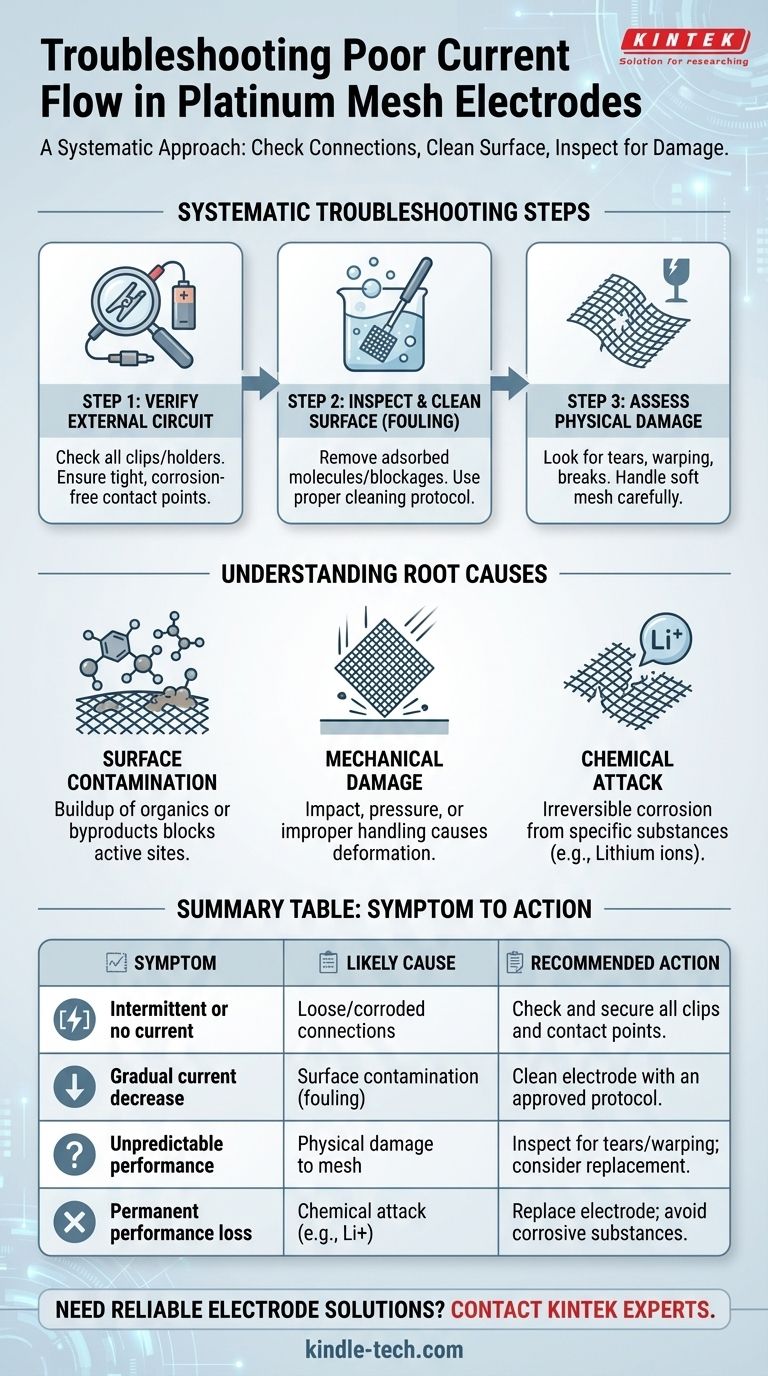
A Systematic Approach to Troubleshooting
When faced with poor current, a methodical process of elimination is the most effective way to identify and solve the problem without causing further damage to this sensitive equipment.
Step 1: Verify the External Circuit
The simplest and most common point of failure is often the physical connection to the electrode. Before assuming the electrode itself is faulty, perform a thorough check of the entire external circuit.
Ensure the clips or holders making contact with the electrode are tight and secure. Look for any signs of oxidation or residue on the contact points, as this can create significant resistance and disrupt current flow.
Step 2: Inspect and Clean the Electrode Surface
If the circuit is secure, the focus shifts to the electrode surface itself. Contamination, also known as fouling, is the next most likely culprit.
Fouling occurs when molecules from your solution (e.g., organic compounds, polymers, or reaction byproducts) adsorb onto the platinum surface. This effectively "blinds" the electrode, blocking sites needed for electron transfer and reducing current. A proper cleaning protocol is necessary to restore the surface.
Step 3: Assess for Physical Damage
Platinum mesh is exceptionally soft and malleable. It can be easily damaged by physical impact, pressure, or improper handling.
Carefully inspect the mesh under good lighting. Look for any tears, warping, or breaks in the fine wires. Any significant deformation can alter the electrode's surface area and create non-uniform current distribution, leading to poor or unpredictable performance.
Understanding the Root Causes of Failure
Troubleshooting solves the immediate problem, but understanding the underlying causes is key to preventing future failures and ensuring the long-term reliability of your measurements.
Surface Contamination (Fouling)
Persistent contact with certain materials is a primary cause of performance degradation. Organic substances, in particular, are notorious for fouling platinum surfaces.
Even the byproducts of your intended electrochemical reaction can build up over time and passivate the electrode. Regular, gentle cleaning between experiments is a critical preventative measure.
Mechanical Damage
The delicate nature of the mesh cannot be overstated. Avoid dropping the electrode, allowing it to strike the sides of the cell, or applying excessive pressure with connecting clips.
Store the electrode in a protective case when not in use. Proper handling is the most effective way to prevent the physical damage that leads to premature failure.
Chemical Attack
While platinum is highly inert, it is not invincible. Certain substances can chemically attack and corrode the electrode, permanently damaging it.
A critical example is lithium ions, which are known to be corrosive to platinum. The use of any lithium-containing electrolyte or solution with a platinum electrode is strictly prohibited and will cause irreversible damage.
Understanding the Pitfalls and When to Stop
Knowing the limits of field repair is just as important as knowing how to perform it. Aggressive troubleshooting can often do more harm than good.
The Limit of Self-Repair
Never attempt to disassemble the electrode or perform significant mechanical repairs yourself. These actions are highly likely to cause more severe and irreparable damage.
If cleaning and connection checks do not solve the problem, and you suspect deeper damage, the correct action is to seek professional service or replace the unit.
Performance Degradation vs. Outright Failure
An electrode does not have to be completely broken to be unusable. Over time, repeated use and cleaning cycles can subtly change the surface morphology, leading to a gradual decline in performance.
If you observe a consistent loss of sensitivity, poor reproducibility, or drifting baselines that cleaning cannot fix, the electrode may have reached the end of its operational life for high-precision work.
The Cost of Inaccurate Data
Continuing to use a faulty or degraded electrode is a false economy. The cost of a new electrode is often minor compared to the cost of time, reagents, and project delays caused by unreliable or non-reproducible experimental data.
Making the Right Decision for Your Goal
Use this checklist to guide your response based on the symptoms you observe.
- If your primary focus is immediate troubleshooting: Systematically check all contact points from the potentiostat to the electrode before taking any other action.
- If you suspect surface contamination: After verifying connections, proceed with a gentle, approved cleaning protocol to restore the active surface area.
- If the electrode is physically damaged or performance is consistently poor: Replace the electrode or consult a professional for repair to ensure the integrity and reproducibility of your results.
A methodical approach to maintenance and troubleshooting is the key to ensuring both the longevity of your electrode and the reliability of your data.
Summary Table:
| Symptom | Likely Cause | Recommended Action |
|---|---|---|
| Intermittent or no current | Loose/corroded connections | Check and secure all clips and contact points |
| Gradual current decrease | Surface contamination (fouling) | Clean electrode with an approved protocol |
| Unpredictable performance | Physical damage to the mesh | Inspect for tears/warping; consider replacement |
| Permanent performance loss | Chemical attack (e.g., from lithium ions) | Replace electrode; avoid corrosive substances |
Struggling with unreliable electrode performance? Don't let faulty equipment compromise your data. KINTEK specializes in high-quality lab equipment and consumables, including durable platinum electrodes designed for precise electrochemical measurements. Our experts can help you select the right tools and provide support to ensure your lab's success. Contact our team today for a consultation and keep your experiments running smoothly.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data



















