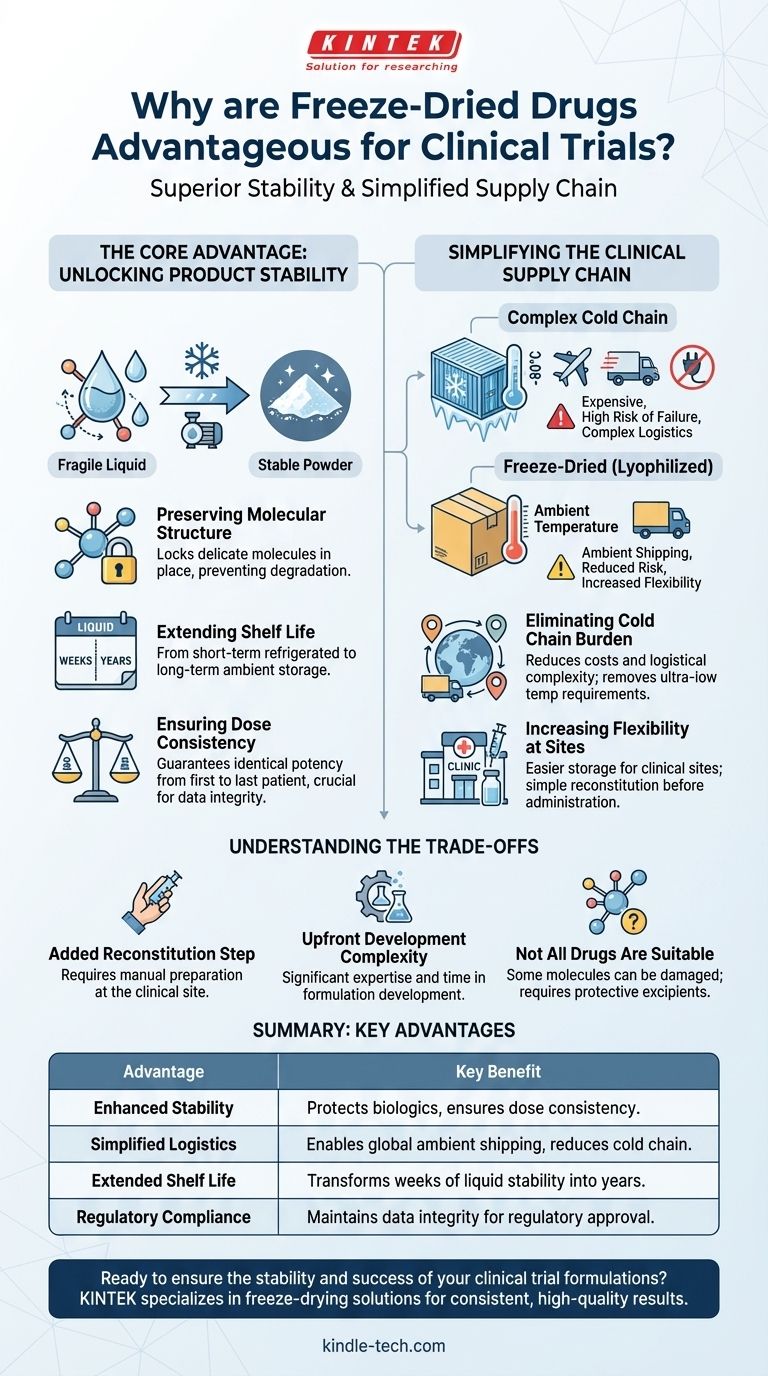
At its core, the advantage of freeze-dried drugs in clinical trials comes down to superior stability and a simplified supply chain. By removing water, the lyophilization process transforms a potentially fragile liquid drug into a stable powder, ensuring the product remains consistent and effective throughout the long and complex duration of a clinical study.
Clinical trials are defined by uncertainty, but the drug product itself cannot be one of the variables. Freeze-drying de-risks the entire process by creating a stable, easy-to-ship formulation that guarantees dose consistency from the first patient to the last, regardless of time or location.
The Core Advantage: Unlocking Product Stability
The primary driver for freeze-drying is to protect the drug from degrading over time. This is especially critical for large-molecule biologics like antibodies and proteins.
Preserving Molecular Structure
Most biologic drugs are delicate structures suspended in water. In a liquid state, these molecules can unfold, clump together (aggregate), or break apart, losing their therapeutic effect.
Freeze-drying gently removes water at low temperatures, essentially locking the drug’s molecular structure in place and preventing these degradation pathways.
Extending Shelf Life from Weeks to Years
A liquid formulation might only be stable for weeks or months, even when refrigerated. This is often insufficient for clinical trials that can span several years.
A freeze-dried product, by contrast, can remain stable at room or refrigerated temperatures for years, ensuring a consistent supply of effective drug for the entire trial.
Ensuring Dose Consistency
The fundamental goal of a clinical trial is to test the safety and efficacy of a drug. This requires that every dose administered is identical in its composition and potency.
By preventing degradation, freeze-drying ensures that the dose given to a patient in year three of a study is the same as the dose given on day one. This data integrity is non-negotiable for regulatory approval.
Simplifying the Clinical Supply Chain
Beyond stability, freeze-drying has a profound impact on the logistics of running a trial, which are often complex and global.
Eliminating the Cold Chain Burden
Many liquid biologics must be shipped and stored frozen, often at ultra-low temperatures (-20°C to -80°C). This "cold chain" is expensive, complex, and carries a high risk of failure.
A power outage or shipping delay can lead to a temperature excursion, forcing the entire shipment of invaluable clinical material to be discarded. Freeze-dried products can often be shipped at ambient temperatures, removing this risk entirely.
Increasing Flexibility at Clinical Sites
Hospital pharmacies and clinics participating in a trial may not have the specialized ultra-low temperature freezers required for frozen drug products.
A stable powder that only requires standard refrigeration or room-temperature storage is far easier for clinical sites to manage. The drug is simply reconstituted with a sterile liquid (like water or saline) just before it is administered to the patient.
Understanding the Trade-offs
While highly advantageous, freeze-drying is not a universal solution and involves important considerations.
The Added Reconstitution Step
A freeze-dried drug cannot be administered directly. It must be reconstituted into a liquid form at the clinical site.
This introduces a manual step that carries a small risk of incorrect preparation, dosing errors, or microbial contamination if aseptic techniques are not strictly followed.
Upfront Development Complexity
Developing a robust and efficient freeze-drying cycle is a complex scientific process. It requires significant time and expertise during the formulation development phase.
This adds time and cost to the early stages of drug development compared to pursuing a simpler liquid formulation.
Not All Drugs Are Suitable
The stresses of the freezing and drying process can damage certain sensitive molecules. Significant formulation work is required to identify protective agents (excipients) that safeguard the drug during lyophilization.
Making the Right Formulation Choice
The decision to freeze-dry a drug depends on balancing the molecule's inherent stability with the logistical demands of your clinical program.
- If your primary focus is a fast, short-term Phase 1 study with a fairly stable molecule: A liquid formulation may be a faster and more cost-effective path to get into the clinic.
- If your primary focus is a long-duration, global Phase 3 trial: The stability and logistical benefits of a freeze-dried product are often essential for ensuring trial integrity and success.
- If you are working with a highly unstable biologic drug: Freeze-drying is frequently the only viable option to create a product with a practical shelf life for clinical or commercial use.
Ultimately, investing in a freeze-dried formulation is an investment in the reliability and logistical feasibility of your entire clinical program.

Summary Table:
| Advantage | Key Benefit |
|---|---|
| Enhanced Stability | Protects delicate biologics from degradation, ensuring dose consistency over years. |
| Simplified Logistics | Reduces or eliminates cold chain requirements, enabling global shipping at ambient temperatures. |
| Extended Shelf Life | Transforms weeks of liquid stability into years of powder stability for long-term trials. |
| Regulatory Compliance | Maintains data integrity by guaranteeing identical potency from first to last patient dose. |
Ready to ensure the stability and success of your clinical trial formulations? KINTEK specializes in providing reliable lab equipment and consumables tailored for pharmaceutical development, including freeze-drying solutions. Our expertise helps you achieve consistent, high-quality results while simplifying your supply chain. Contact us today to discuss how we can support your clinical trial needs!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Anti-Cracking Press Mold for Lab Use
- Single Punch Tablet Press Machine and Mass Production Rotary Tablet Punching Machine for TDP
People Also Ask
- What precautions should be taken when using a laboratory freeze dryer? Essential Steps for Reliable Lyophilization
- What is a freeze dryer and what does it do? Preserve Delicate Materials with Sublimation
- What are the main components of a laboratory freeze dryer? A Guide to the 5 Essential Systems
- What is the purpose of laboratory freeze drying? Preserve Sensitive Drugs & Biologics for Stability
- Why is freeze drying considered more effective than ordinary drying? Preserve Structure, Nutrients & Flavor



















