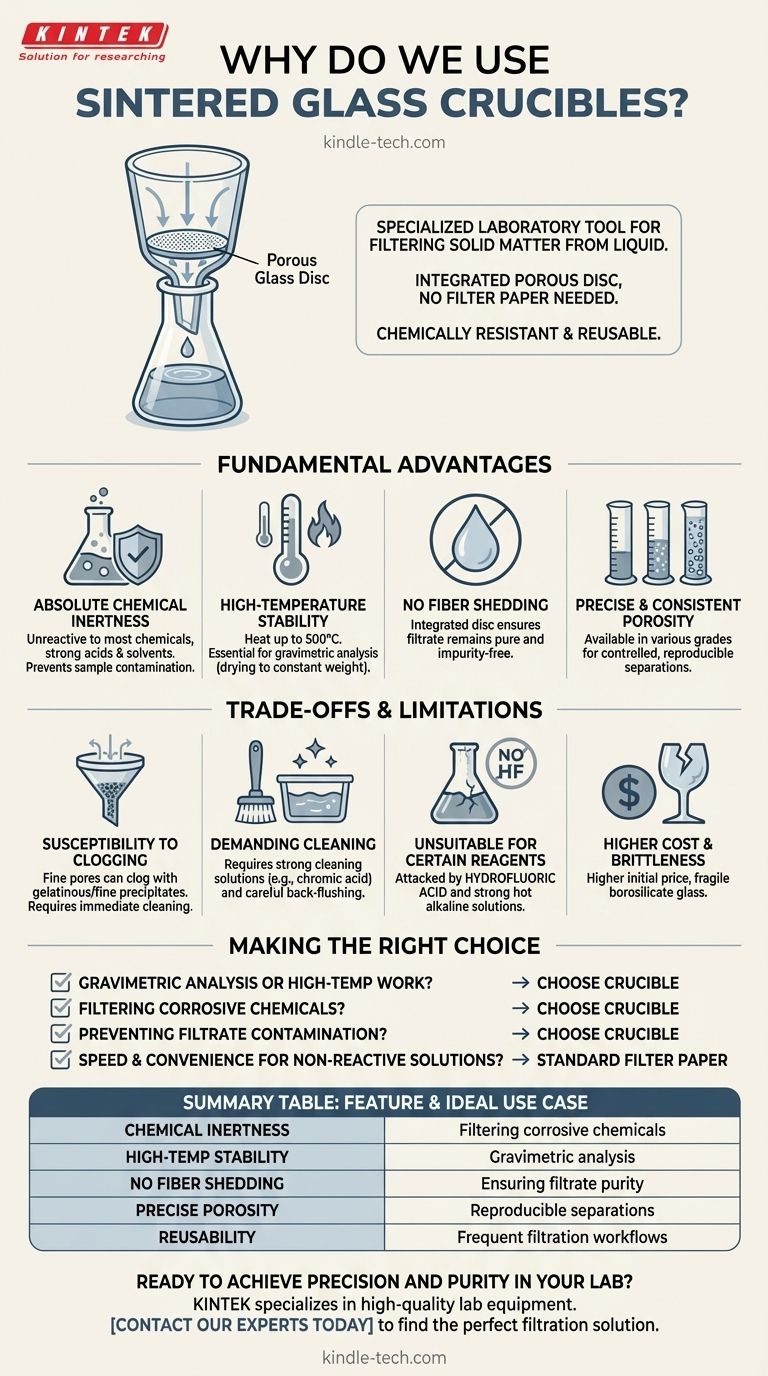
At its core, a sintered glass crucible is a specialized laboratory tool for filtering solid matter from a liquid. It functions like a funnel but features a built-in, porous glass disc. This integrated design eliminates the need for filter paper, providing a chemically resistant and reusable solution for precise filtration tasks.
The decision to use a sintered glass crucible over simpler methods like filter paper is driven by the need for chemical inertness, high-temperature stability, and the prevention of sample contamination in demanding analytical procedures.

The Fundamental Advantages Over Other Methods
While filter paper is common, a sintered glass crucible is chosen when the experimental conditions demand higher performance and purity. Its construction from borosilicate glass provides several key benefits.
Absolute Chemical Inertness
The crucible is made of glass, which is unreactive to most chemical solutions, including strong acids and solvents. This is critical in analyses where reactive filter paper (made of cellulose) would be degraded or contaminate the sample.
High-Temperature Stability
You can heat a sintered glass crucible to very high temperatures (typically up to 500°C). This allows for the drying of a captured precipitate to a constant weight, a fundamental step in gravimetric analysis that is impossible with combustible filter paper.
No Fiber Shedding
Filter papers can shed microscopic cellulose fibers, contaminating the filtrate (the liquid that passes through). A sintered glass crucible's solid, integrated disc ensures that the filtrate remains free of any such impurities.
Precise and Consistent Porosity
These crucibles are manufactured in various porosity grades, from coarse to very fine. This allows the user to select the precise pore size needed to reliably capture a specific precipitate, offering more control and reproducibility than standard filter paper.
Reusability and Longevity
With proper care, a sintered glass crucible can be thoroughly cleaned and reused hundreds of times. This makes it a more sustainable and cost-effective choice in the long run for labs that perform filtration frequently.
Understanding the Trade-offs and Limitations
Despite its advantages, the sintered glass crucible is not the right tool for every situation. Understanding its limitations is key to using it effectively.
Susceptibility to Clogging
The fine pores, especially in finer grades, can become permanently clogged by gelatinous or very fine precipitates. This can render the crucible useless if not cleaned immediately and correctly.
Demanding Cleaning Procedures
Cleaning is far more involved than simply discarding a piece of filter paper. It often requires soaking in strong cleaning solutions (like chromic acid or Nochromix) and careful back-flushing to dislodge trapped particles.
Unsuitability for Certain Reagents
While highly inert, borosilicate glass will be attacked by hydrofluoric acid and strong, hot alkaline solutions. Using these chemicals will damage the sintered disc and ruin the crucible.
Higher Initial Cost and Brittleness
The initial purchase price is significantly higher than a pack of filter paper. Furthermore, being made of glass, it is fragile and can be easily broken by mishandling or severe thermal shock.
Making the Right Choice for Your Filtration Task
Choose your filtration method based on the specific demands of your experiment.
- If your primary focus is gravimetric analysis or high-temperature work: The crucible's ability to be heated to a constant mass makes it the only viable choice.
- If your primary focus is filtering corrosive chemicals: Its chemical inertness provides a reliability that filter paper cannot match.
- If your primary focus is preventing filtrate contamination: The crucible's solid, no-shedding design ensures the absolute purity of the liquid passing through.
- If your primary focus is speed and convenience for non-reactive solutions: Standard filter paper in a funnel is often faster, cheaper, and requires no cleanup.
Ultimately, selecting a sintered glass crucible is a deliberate choice for achieving precision, purity, and durability in demanding chemical environments.
Summary Table:
| Feature | Advantage | Ideal Use Case |
|---|---|---|
| Chemical Inertness | Resists strong acids/solvents | Filtering corrosive chemicals |
| High-Temperature Stability | Can be dried at high heat (up to 500°C) | Gravimetric analysis |
| No Fiber Shedding | Prevents sample contamination | Ensuring filtrate purity |
| Precise Porosity Grades | Consistent particle retention | Reproducible separations |
| Reusability | Cost-effective over time | Frequent filtration workflows |
Ready to achieve precision and purity in your lab filtration?
KINTEK specializes in high-quality lab equipment, including sintered glass crucibles designed for demanding analytical procedures. Our products ensure chemical inertness, thermal stability, and reliable performance for your gravimetric analysis and sensitive separations.
Contact our experts today to find the perfect filtration solution for your laboratory's needs!
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- What precautions should be taken when using a crucible? Essential Steps for Safety and Accuracy
- What is a crucible material for a furnace? A Guide to Choosing the Right High-Temperature Container
- Why use high-purity alumina crucibles for RPPO calcination? Ensure Stoichiometric Purity at 1150°C
- What are the benefits of using an alumina crucible with a lid for TiB2 nanopowder heat treatment? Ensure High Purity
- Why are alumina crucibles selected for wood-plastic composite tests? Ensure Precision at 1000°C



















