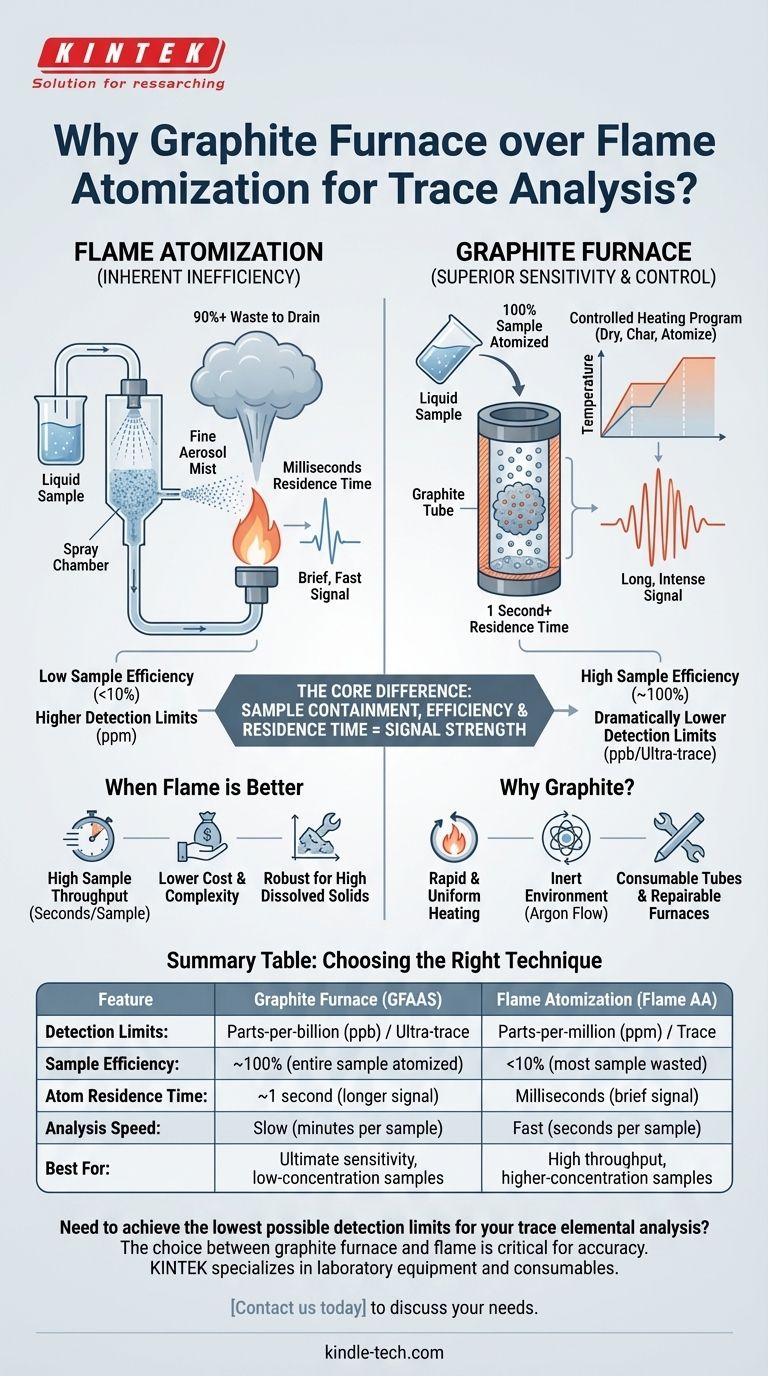
In analytical chemistry, a graphite furnace is often preferred over a flame for atomization because it offers vastly superior sensitivity and much lower detection limits. The furnace achieves this by efficiently atomizing the entire sample and containing the resulting cloud of atoms within a confined light path for an extended period, which maximizes the measured absorbance signal.
The fundamental difference lies in efficiency and residence time. A flame system is inefficient, wasting most of the sample and offering only a fleeting moment for measurement. A graphite furnace provides a highly controlled environment that atomizes nearly 100% of the sample and holds the atoms in the light path, dramatically increasing signal strength.

The Core Difference: Sample Containment and Efficiency
The primary reason for the graphite furnace's superiority in trace analysis comes down to how it handles the sample compared to a flame.
The Inefficiency of Flame Atomization
In Flame Atomic Absorption (Flame AA), the liquid sample is pulled into a spray chamber. This process is inherently wasteful.
A large portion of the sample, typically over 90%, condenses on the chamber walls and goes to drain. Only a fine aerosol mist makes it to the flame, where the atoms are created. These atoms then pass through the instrument's light path very quickly, offering a very brief window for measurement.
The Total Efficiency of the Graphite Furnace
Graphite Furnace AA (GFAAS) uses a completely different approach. A small, precise volume of the sample is placed directly inside the graphite tube.
The furnace then heats in a controlled, programmed sequence. This process atomizes the entire sample, not just a small fraction. Because the atomization occurs within the small, enclosed tube, the resulting atoms are trapped in the instrument's light path for a second or more—a significantly longer residence time than in a flame.
Maximizing the Analytical Signal
This combination of 100% sample atomization and long residence time is the key to the graphite furnace's power. By keeping a dense cloud of atoms in the light path for a longer duration, the instrument can measure a much stronger and more integrated absorbance signal, leading to dramatically lower detection limits.
Why Graphite is the Ideal Furnace Material
The choice of graphite is not arbitrary. Its unique physical properties make it perfectly suited for this application.
Rapid and Uniform Heating
Graphite has a modest thermal capacity and allows for extremely fast heating speeds. This enables the precise temperature control needed to dry, char, and then explosively atomize the sample.
Furthermore, a well-designed furnace provides excellent temperature uniformity, ensuring that all parts of the sample are atomized simultaneously. This creates a sharp, narrow, and intense signal peak, which is ideal for quantification.
Creating an Inert Environment
Graphite furnaces operate with a constant flow of an inert gas, such as argon. This prevents oxygen from entering the tube, which would otherwise incinerate the sample and rapidly degrade the hot graphite. This inert environment is critical for achieving clean, reproducible atomization.
Practical and Physical Advantages
Graphite has a low density, is easy to machine, and is relatively inexpensive. Furnaces are often designed to be easily repairable, and the tubes themselves are consumables that can be protected with sacrificial layers to extend their lifespan, managing the operational costs of the technique.
Understanding the Trade-offs: When a Flame is Better
Despite its sensitivity, the graphite furnace is not always the best choice. It comes with significant trade-offs compared to the simplicity of a flame.
Speed and Sample Throughput
A flame system is a true workhorse. It can analyze a sample in a matter of seconds. In contrast, a graphite furnace program takes several minutes per sample due to the necessary heating and cooling cycles. For labs with high sample loads and less stringent sensitivity requirements, Flame AA is far more productive.
Cost and Complexity
Graphite furnace systems are more expensive to purchase and operate. The graphite tubes have a limited lifetime and must be replaced regularly, adding to the consumable cost. The method also requires a higher level of operator skill to develop methods and troubleshoot interferences.
Matrix Interferences
While incredibly sensitive, GFAAS can be more susceptible to chemical and matrix interferences than Flame AA. Overcoming these interferences often requires careful optimization of the temperature program and the use of chemical "matrix modifiers."
Making the Right Choice for Your Analysis
Selecting the correct atomization technique is a critical decision driven by your analytical needs.
- If your primary focus is ultimate sensitivity and low detection limits: The graphite furnace is the superior choice, making it essential for trace and ultra-trace elemental analysis in fields like environmental science and clinical diagnostics.
- If your primary focus is high sample throughput and cost-effectiveness: Flame atomization is far more efficient for analyzing hundreds of samples per day, especially when element concentrations are in the parts-per-million (ppm) range.
- If your primary focus is analyzing samples with high dissolved solids: A flame system is often more robust and less prone to the physical and chemical interferences that high-matrix samples can cause in a graphite furnace.
Ultimately, the choice between flame and furnace is a strategic decision based on the specific analytical goals of concentration, speed, and precision.
Summary Table:
| Feature | Graphite Furnace (GFAAS) | Flame Atomization (Flame AA) |
|---|---|---|
| Detection Limits | Parts-per-billion (ppb) / Ultra-trace | Parts-per-million (ppm) / Trace |
| Sample Efficiency | ~100% (entire sample atomized) | <10% (most sample wasted) |
| Atom Residence Time | ~1 second (longer signal) | Milliseconds (brief signal) |
| Analysis Speed | Slow (minutes per sample) | Fast (seconds per sample) |
| Best For | Ultimate sensitivity, low-concentration samples | High throughput, higher-concentration samples |
Need to achieve the lowest possible detection limits for your trace elemental analysis?
The choice between a graphite furnace and a flame is critical for your lab's accuracy and efficiency. KINTEK specializes in laboratory equipment and consumables, providing the right tools for your specific analytical challenges.
Let our experts help you select the ideal atomization system for your needs. Contact us today to discuss how our solutions can enhance your lab's capabilities in environmental, clinical, or materials analysis.
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- Why graphite is used in furnace? Achieve Superior Heat Treatment & Energy Efficiency
- Does graphite have a melting point? Unlocking the Extreme Heat Resistance of Graphite
- What is the purpose of a graphite furnace? Achieve Extreme Temperatures for Advanced Materials
- What is the temperature of a graphite furnace? Achieve Extreme Heat Up to 3000°C
- What is the temperature range of a graphite furnace? Unlock up to 3000°C for advanced materials processing.



















