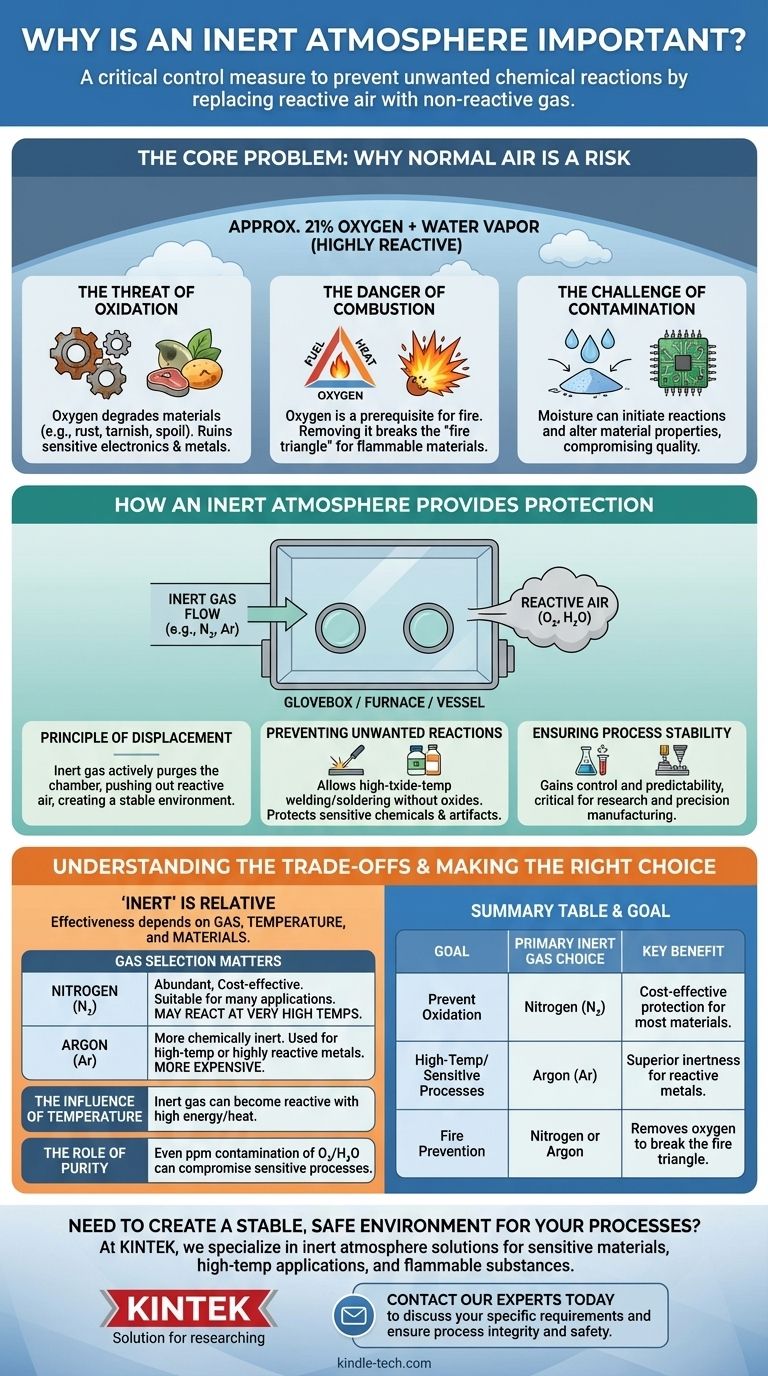
An inert atmosphere is a critical control measure used to prevent unwanted chemical reactions. By replacing the reactive air in a workspace—primarily its oxygen and water vapor—with a non-reactive gas, you protect materials from degradation, ensure the purity of a process, and eliminate the risk of fire or explosion.
The air around us is not neutral; it's a reactive chemical agent that can damage sensitive materials and disrupt delicate processes. An inert atmosphere serves as a protective shield, creating a stable environment where work can be performed without the risk of unwanted atmospheric interference.

The Core Problem: Why Normal Air is a Risk
To understand the solution, we must first appreciate the problem. The standard atmosphere we live in is approximately 21% oxygen and contains variable amounts of water vapor, both of which are highly reactive.
The Threat of Oxidation
Oxygen is aggressive. It readily reacts with many materials in a process called oxidation, which degrades them.
This is the same chemical reaction that causes iron to rust, copper to tarnish, and foods to spoil. In a technical or manufacturing setting, oxidation can ruin sensitive electronics, weaken metals, and alter the chemical composition of a product.
The Danger of Combustion
The presence of oxygen is a prerequisite for fire. For combustion to occur, three things are needed: fuel, heat, and an oxidizing agent (typically oxygen).
By removing oxygen from the equation, you break this "fire triangle." This is essential when working with flammable solvents, fine metal powders, or other materials that could ignite or explode in a normal atmosphere.
The Challenge of Contamination
Beyond oxygen, other atmospheric components like moisture can act as contaminants. Water vapor can initiate unwanted reactions or be absorbed by hygroscopic materials, altering their properties and compromising the quality of the final product.
How an Inert Atmosphere Provides Protection
Creating an inert atmosphere involves actively displacing the ambient air in a sealed environment—such as a glovebox, furnace, or reaction vessel—and replacing it with a gas that will not react with the materials inside.
The Principle of Displacement
The fundamental mechanism is simple: a steady flow of inert gas is used to purge the chamber, pushing the lighter, reactive air out. The result is an internal atmosphere composed almost entirely of the stable, non-reactive gas.
Preventing Unwanted Reactions
With oxygen and moisture removed, the primary drivers of degradation are gone. This allows for processes like high-temperature welding or soldering without the formation of oxides that weaken the bond. It also allows for the long-term storage of sensitive chemicals or artifacts.
Ensuring Process Stability
By removing the variable of atmospheric reactivity, you gain greater control and predictability over your process. This stability is critical in scientific research and high-precision manufacturing, where even minor, unintended reactions can lead to failure.
Understanding the Trade-offs: "Inert" is Relative
The term "inert" is not absolute. The effectiveness of an inert atmosphere depends on the specific gas used, the temperature of the process, and the materials involved.
Gas Selection Matters
The most common inert gases are nitrogen (N₂) and argon (Ar). Nitrogen is abundant and cost-effective, making it suitable for many applications. However, at very high temperatures, it can react with certain metals to form nitrides.
Argon is more chemically inert than nitrogen and is often used for high-temperature processes or with highly reactive metals where nitrogen is unsuitable. It is, however, significantly more expensive.
The Influence of Temperature
A gas that is inert at room temperature can become reactive when enough energy, such as high heat, is introduced. This is why material and temperature are critical factors when selecting the right inert gas for a furnace or welding application.
The Role of Purity
The purity of the inert gas is paramount. Even a few parts-per-million of oxygen or moisture contamination in the gas supply can be enough to compromise a highly sensitive process.
Making the Right Choice for Your Goal
Selecting the right atmospheric controls depends entirely on your objective, balancing cost against the required level of protection.
- If your primary focus is general oxidation prevention: Nitrogen is often the most practical and cost-effective choice for many materials and processes.
- If your primary focus is high-temperature or highly sensitive materials: Argon provides a superior degree of inertness, ensuring protection when even nitrogen might react.
- If your primary focus is safety and fire prevention: Displacing oxygen with any common inert gas is the fundamental strategy to eliminate the risk of combustion.
By deliberately controlling the atmosphere, you gain ultimate control over the integrity, quality, and safety of your work.
Summary Table:
| Goal | Primary Inert Gas Choice | Key Benefit |
|---|---|---|
| Prevent Oxidation | Nitrogen (N₂) | Cost-effective protection for most materials |
| High-Temperature/Sensitive Processes | Argon (Ar) | Superior inertness for reactive metals |
| Fire Prevention | Nitrogen or Argon | Removes oxygen to break the fire triangle |
Need to create a stable, safe environment for your processes?
At KINTEK, we specialize in providing the right lab equipment and expertise to implement effective inert atmosphere solutions—whether you're working with sensitive materials, high-temperature applications, or flammable substances. Our team can help you select the optimal system to protect your work from oxidation, contamination, and combustion risks.
Contact our experts today to discuss your specific requirements and ensure the integrity and safety of your laboratory processes!
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are inert atmosphere conditions? Control Chemical Reactions and Ensure Safety
- What products use annealing? Enhance Formability and Durability in Metal Manufacturing
- What is annealing in air atmosphere? A Simple Guide to Cost-Effective Metal Softening
- What are the two types of exothermic atmospheres and their applications? Rich vs. Lean Atmospheres Explained
- Why is an atmosphere sintering furnace used for post-annealing ZnO ceramics? Optimize Conductivity & Density
- How do controlled atmosphere pyrolysis furnaces ensure product diversity? Unlock High-Value Coal Gangue Utilization
- How to prevent oxides during brazing? Key Methods for Oxide-Free Joints
- What is the function of inert atmosphere heating equipment in preparing 70Li2S·(30-x)P2S5·xSeS2 glass-ceramics?



















