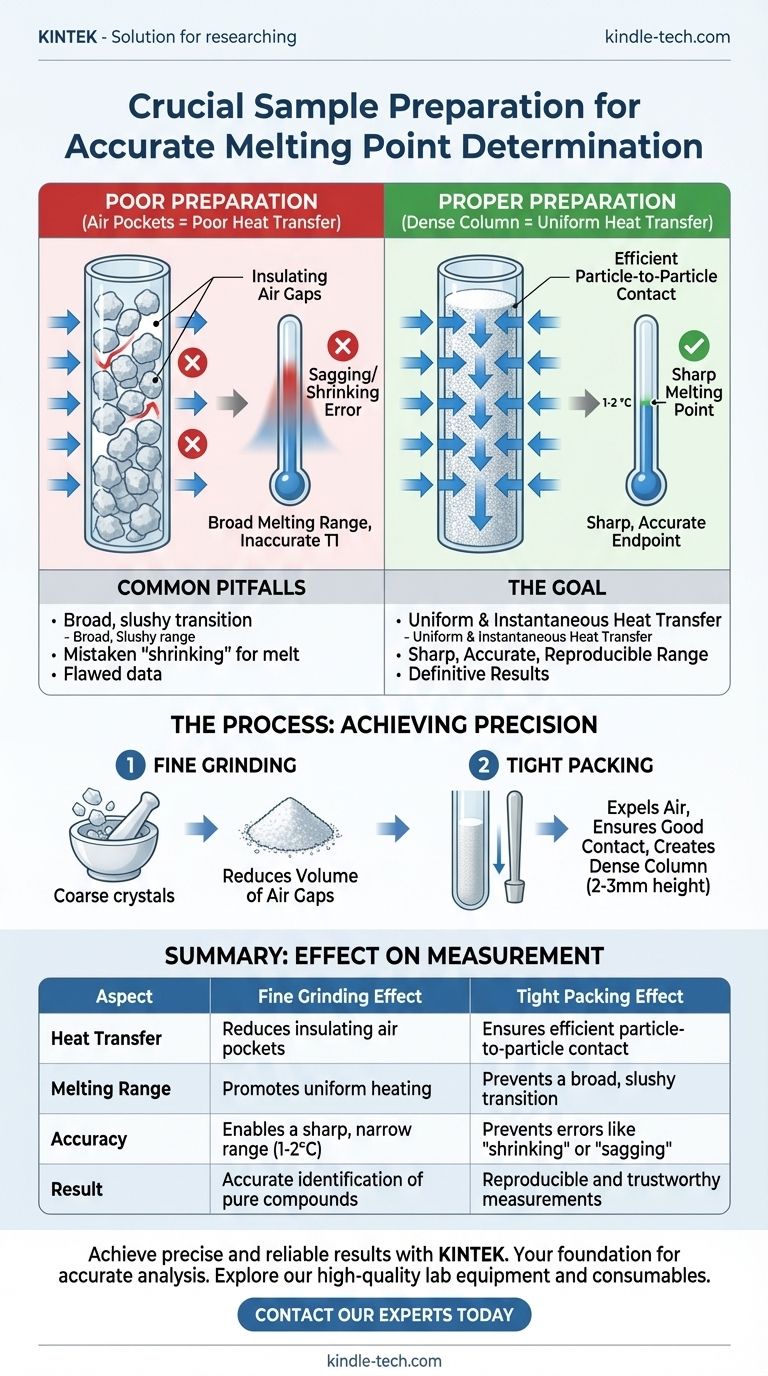
In short, grinding your sample finely and packing it tightly are critical for ensuring rapid, uniform heat transfer throughout the solid. This preparation minimizes air pockets and creates a dense column, which is the key to observing a sharp, accurate, and reproducible melting point range.
A melting point determination is not just a measurement of temperature; it's an observation of a physical change. Your goal is to prepare a sample where every particle reaches its melting temperature at nearly the same instant, and proper preparation is the only way to achieve this.

The Goal: Uniform and Instantaneous Heat Transfer
The entire melting point apparatus is designed to heat a small sample slowly and evenly. However, this design assumes the sample itself can conduct that heat efficiently. Your preparation technique determines whether this assumption holds true.
The Role of Fine Grinding
Grinding the sample into a fine, consistent powder is the first step. This directly addresses the problem of air, which is an excellent insulator and the enemy of good heat transfer.
Large, irregular crystals trap significant air pockets between them. When you heat the sample, this trapped air slows the transfer of heat from the outside of the sample to the inside, creating a temperature gradient.
By grinding the material, you create smaller particles that fit together more closely, drastically reducing the volume of these insulating air gaps and ensuring the entire sample heats more uniformly.
The Role of Tight Packing
Packing the finely ground powder tightly into the capillary tube is the second critical step. This physically expels the remaining air and ensures good contact between the particles.
A tightly packed column of solid acts as a single, dense unit. Heat is conducted efficiently from the wall of the glass capillary, through the outer particles, and into the center of the sample.
If the sample is loosely packed, particles at the edge near the glass will heat up and melt before the particles in the thermally-isolated center. This results in a slow, staggered melt over a wide temperature range, not the sharp point you need.
Common Pitfalls from Poor Sample Preparation
Failing to properly grind and pack your sample will not just give you a slightly "off" number; it will produce fundamentally flawed data.
The "Shrinking" or "Sagging" Error
A loosely packed solid will often shrink or contract within the tube as it is heated, well before any melting has occurred. This is a purely physical process of the powder settling.
An operator can easily mistake this movement for the beginning of the melt (the "T1" temperature). This leads to an erroneously low and inaccurate starting point for the melting range.
A Broad and Inaccurate Melting Range
The hallmark of a pure compound measured with good technique is a sharp, narrow melting range (typically 1-2 °C).
Poor heat transfer caused by air gaps means some parts of the sample will be fully molten while other parts are still solid. The result is a broad, slushy transition that can span many degrees, making it impossible to determine an accurate endpoint and falsely suggesting the sample is impure.
How to Apply This to Your Measurement
Following a systematic process for sample preparation is the difference between ambiguous data and a definitive result.
- If your primary focus is accuracy (e.g., identifying a pure unknown): You must grind the sample to a fine powder and pack it tightly to a height of 2-3 mm. This is the only way to achieve the sharp, narrow melting range characteristic of a pure substance.
- If you observe the sample shrinking or sagging: This is a clear sign it is not packed tightly enough. Prepare a new sample, and after loading the powder, tap the capillary tube firmly on a hard surface to compact the solid into a dense column.
- If you observe a very broad melting range (>3 °C): Your sample is either impure or your preparation was poor. Re-grind the material to a finer consistency and re-pack it tightly to rule out technical error.
Ultimately, the few moments spent on meticulous sample preparation are the foundation of a reliable and trustworthy melting point measurement.
Summary Table:
| Aspect | Fine Grinding Effect | Tight Packing Effect |
|---|---|---|
| Heat Transfer | Reduces insulating air pockets | Ensures efficient particle-to-particle contact |
| Melting Range | Promotes uniform heating | Prevents a broad, slushy transition |
| Accuracy | Enables a sharp, narrow range (1-2°C) | Prevents errors like 'shrinking' or 'sagging' |
| Result | Accurate identification of pure compounds | Reproducible and trustworthy measurements |
Achieve precise and reliable results in your laboratory. Proper sample preparation is the foundation of accurate analysis. KINTEK specializes in providing high-quality lab equipment and consumables, including reliable melting point apparatus and sample preparation tools, to support your laboratory's needs for precise and reproducible measurements. Contact our experts today to find the perfect equipment for your application and enhance your lab's efficiency and data quality.
Visual Guide
Related Products
- Lab-Scale Vacuum Induction Melting Furnace
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Special Heat Press Mold for Lab Use
- Laboratory Vibratory Sieve Shaker Machine Slap Vibrating Sieve
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
People Also Ask
- What principle is used to generate heat in a vacuum induction melting furnace? Achieve Clean, Efficient Metal Melting
- What types of metals are typically processed in a vacuum induction melting furnace? High-Purity Alloys for Critical Applications
- How does induction work in a vacuum? Achieve Ultra-Pure Metal Melting with VIM
- What is the primary function of a vacuum induction melting furnace? Melt High-Purity Metals with Precision
- What is VIM in metallurgy? A Guide to Vacuum Induction Melting for High-Performance Alloys



















