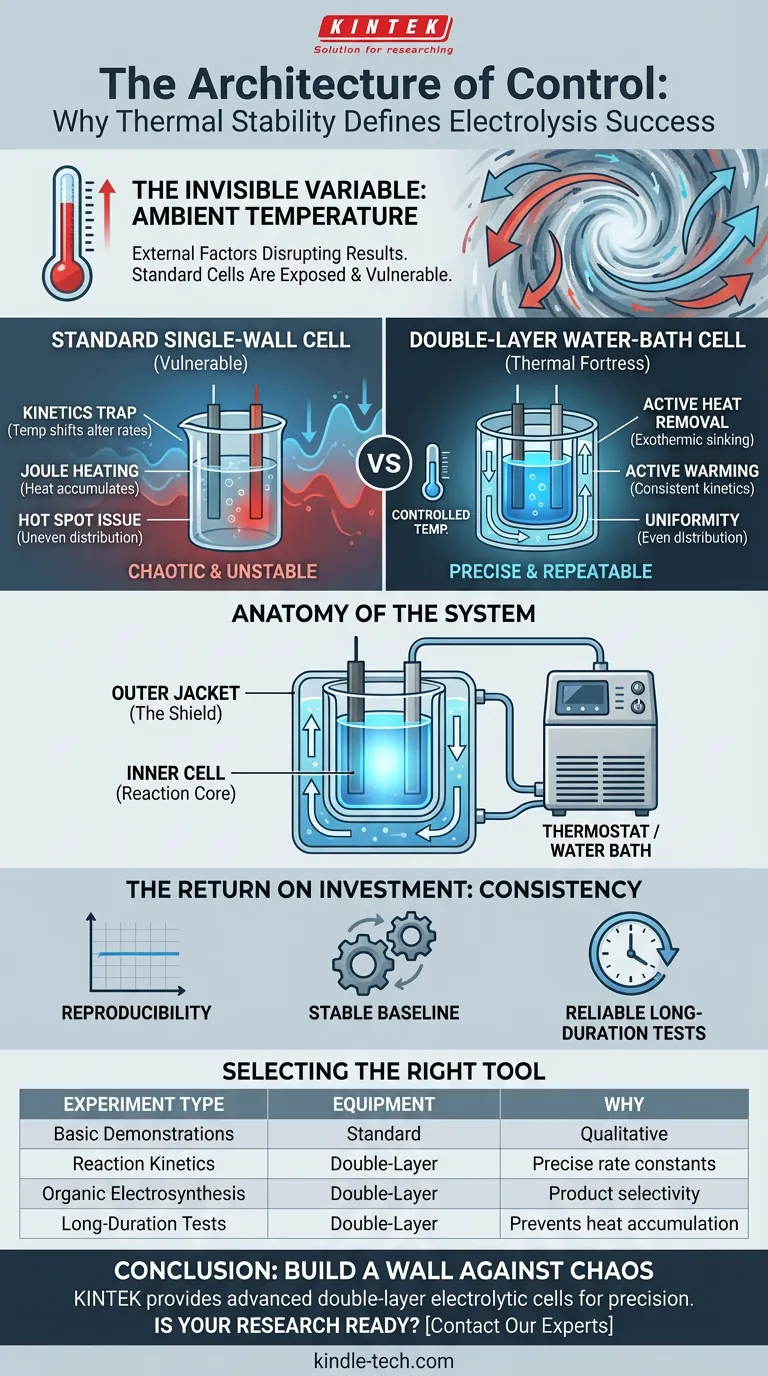
The Invisible Variable
In scientific research, we are obsessed with the variables we can see.
We meticulously measure voltage. We weigh catalysts to the microgram. We purify electrolytes until they are spotless. We treat these visible inputs as the gods of our experiment.
But often, the most destructive variable is the one we ignore: Ambient temperature.
Imagine running an electrolysis experiment on a Tuesday morning when the lab is cool. You get a yield of 85%. You repeat it on a Friday afternoon when the sun is hitting the bench and the room is five degrees warmer. The yield drops to 70%, or perhaps side products appear.
You haven't changed the chemistry. The room changed the chemistry for you.
This is the fundamental problem of the standard electrolytic cell. It is exposed. It is vulnerable to the chaos of the environment.
The Engineering of Isolation
The solution to this vulnerability is not more complex chemistry. It is better engineering.
Enter the Double-Layer Water-Bath Electrolytic Cell.
At a glance, it looks like simple glassware. But functionally, it is a thermal fortress. It is designed to do one thing: eliminate temperature as a variable.
Anatomy of the System
The design is elegant in its simplicity, consisting of two distinct chambers:
- The Inner Cell (The Reaction Core): This is where the science happens. It holds your electrolyte, anode, and cathode. To the chemical reaction, this is the entire universe.
- The Outer Jacket (The Shield): This is a sealed layer surrounding the inner cell. It does not touch the chemicals. Instead, it circulates a liquid—usually water—controlled by an external thermostat.
This "moat" of circulating liquid isolates the reaction from the lab environment. It creates a micro-climate where the laws of thermodynamics are strictly enforced by the researcher, not the weather.
Why Standard Cells Fail at Precision
To understand why the double-layer design is necessary, we have to look at what happens in a standard, single-wall cell.
1. The Kinetics Trap
Reaction rates are governed by temperature. A shift of just a few degrees can significantly alter the speed of electrolysis. In a standard cell, you cannot know if a spike in current is due to your catalyst working better or simply because the solution got warmer.
2. The Joule Heating Problem
Electrolysis is rarely energy neutral. Pushing current through a resistive solution generates heat (Joule heating).
In a standard cell, this heat accumulates. The solution warms up as the experiment progresses. This means the conditions at Minute 1 are completely different from the conditions at Minute 60. You aren't measuring a steady state; you are measuring a moving target.
3. The Hot Spot Issue
Without active circulation, heat distributes unevenly. You get "hot spots" on the electrode surface. This leads to inconsistent reaction rates across the same electrode, degrading efficiency and shortening the lifespan of your materials.
The Return on Investment: Consistency
The double-layer cell is not just a vessel; it is a tool for reproducibility.
When you utilize a jacketed cell connected to a water bath, you gain three active advantages:
- Active Heat Removal: If your reaction is exothermic (heat-generating), the flowing water acts as a heat sink, instantly carrying away excess energy to maintain a flat baseline.
- Active Warming: If you need to test reaction kinetics at exactly 60°C, the jacket maintains that temperature evenly, wrapping the reaction in a blanket of thermal consistency.
- Uniformity: Because the water circulates, the temperature is the same at the bottom of the cell as it is at the top.
Selecting the Right Tool
Not every experiment requires this level of architectural control. But for those pushing the boundaries of science, the choice becomes clear.
| Experiment Type | Recommended Equipment | Why? |
|---|---|---|
| Basic Demonstrations | Standard Single-Walled Cell | Cost-effective; thermal drift is negligible for qualitative results. |
| Reaction Kinetics | Double-Layer Cell | precise temperature is required to calculate rate constants. |
| Organic Electrosynthesis | Double-Layer Cell | Temperature often dictates product selectivity (avoiding byproducts). |
| Long-Duration Tests | Double-Layer Cell | Prevents heat accumulation over hours of operation. |
Conclusion
Great science isn't just about discovery; it's about repeatability. If you cannot repeat it, you didn't learn it.
The double-layer water-bath electrolytic cell is the physical manifestation of discipline. It acknowledges that the environment is chaotic, and it builds a wall against that chaos to ensure that when your results change, it’s because you discovered something new—not because the room got hot.
At KINTEK, we understand that your equipment is the foundation of your data. We provide advanced double-layer electrolytic cells designed to give you that necessary control.
Is your research ready for the next level of precision?
Visual Guide
Related Products
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Side Window Optical Electrolytic Electrochemical Cell
Related Articles
- Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- Exploring the Multifunctional Electrolytic Cell Water Bath: Applications and Benefits




















